Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery
- PMID: 23231989
- PMCID: PMC4433055
- DOI: 10.1109/RBME.2012.2211076
Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery
Abstract
Since the discovery of functional connectivity in fMRI data (i.e., temporal correlations between spatially distinct regions of the brain) there has been a considerable amount of work in this field. One important focus has been on the analysis of brain connectivity using the concept of networks instead of regions. Approximately ten years ago, two important research areas grew out of this concept. First, a network proposed to be "a default mode of brain function" since dubbed the default mode network was proposed by Raichle. Secondly, multisubject or group independent component analysis (ICA) provided a data-driven approach to study properties of brain networks, including the default mode network. In this paper, we provide a focused review of how ICA has contributed to the study of intrinsic networks. We discuss some methodological considerations for group ICA and highlight multiple analytic approaches for studying brain networks. We also show examples of some of the differences observed in the default mode and resting networks in the diseased brain. In summary, we are in exciting times and still just beginning to reap the benefits of the richness of functional brain networks as well as available analytic approaches.
Figures
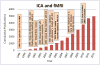
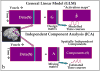




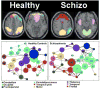




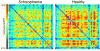
References
-
- Bell AJ, Sejnowski TJ. An information maximisation approach to blind separation and blind deconvolution. Neural Computing. 1995;7:1129–1159. - PubMed
-
- Calhoun VD, Adali T, Mc Ginty V, Pekar JJ, Watson T, Pearlson GD. fMRI Activation In A Visual-Perception Task: Network Of Areas Detected Using The General Linear Model And Independent Component Analysis. NeuroImage. 2001;14:1080–1088. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

