Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial)
- PMID: 23232151
- PMCID: PMC3672620
- DOI: 10.1186/cc11895
Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial)
Abstract
Introduction: Several observational studies suggest that statins modulate the pathophysiology of sepsis and may prevent its progression. The aim of this study was to determine if the acute administration of atorvastatin reduces sepsis progression in statin naïve patients hospitalized with sepsis.
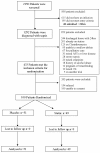
Methods: A single centre phase II randomized double-blind placebo-controlled trial. Patients with sepsis were randomized to atorvastatin 40 mg daily or placebo for the duration of their hospital stay up to a maximum of 28-days. The primary end-point was the rate of sepsis progressing to severe sepsis during hospitalization.
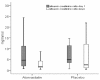
Results: 100 patients were randomized, 49 to the treatment with atorvastatin and 51 to placebo. Patients in the atorvastatin group had a significantly lower conversion rate to severe sepsis compared to placebo (4% vs. 24% p = 0.007.), with a number needed to treat of 5. No significant difference in length of hospital stay, critical care unit admissions, 28-day and 12-month readmissions or mortality was observed. Plasma cholesterol and albumin creatinine ratios were significantly lower at day 4 in the atorvastatin group (p < 0.0001 and p = 0.049 respectively). No difference in adverse events between the two groups was observed (p = 0.238).
Conclusions: Acute administration of atorvastatin in patients with sepsis may prevent sepsis progression. Further multi-centre trials are required to verify these findings.
Trial registration: International Standard Randomized Control Trial Registry ISRCTN64637517.
Figures
Comment in
-
A new role for statins in sepsis.Crit Care. 2013 Jan 17;17(1):105. doi: 10.1186/cc11907. Crit Care. 2013. PMID: 23324213 Free PMC article.
References
-
- National Audit Office. The management and control of hospital acquired infection in acute NHS Trusts in England. 2000. http://www.nao.org.uk/publications/9900/hospital_acquired_infection.aspx - PubMed
-
- Le G Jr, Alberti C, Brun BC. Epidemiology of infection and sepsis in intensive care unit patients. Bull Acad Natl Med. 2004;16:1115–1125. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

