Does oral prednisolone increase the efficacy of subsequent nasal steroids in treating nasal polyposis?
- PMID: 23232195
- PMCID: PMC3903103
- DOI: 10.2500/ajra.2012.26.3820
Does oral prednisolone increase the efficacy of subsequent nasal steroids in treating nasal polyposis?
Abstract
Background: Although combined oral and nasal steroid therapy is widely used in nasal polyposis, a subset of patients show an unfavorable therapeutic outcome. This study aimed to evaluate whether oral prednisolone produces any additive effects on subsequent nasal steroid therapy and to evaluate if any clinical variables can predict therapeutic outcome.
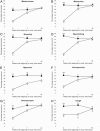
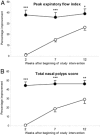
Methods: Using a 3:2 randomization ratio, 67 patients with nasal polyposis received 50 mg of prednisolone and 47 patients received placebo daily for 2 weeks, followed by mometasone furoate nasal spray (MFNS) at 200 micrograms twice daily for 10 weeks. Clinical response was evaluated by nasal symptom score (NSS), peak expiratory flow index (PEFI), and total nasal polyps score (TNPS). Potential predictor variables were assessed by clinical history, nasal endoscopy, allergy skin test, and sinus radiography.
Results: At the end of the 2-week oral steroid phase, the prednisolone group showed significantly greater improvements in all nasal symptoms, nasal airflow, and polyp size than the placebo group. In the nasal steroid phase, while the MFNS maintained the outcome improvements in the prednisolone group, all outcome variables in the placebo group showed continuing improvements. At the end of the nasal steroid phase, there were no significant differences of most outcome improvements between the two groups, except in hyposmia, PEFI, and TNPS (p = 0.049, p = 0.029, and p = 0.005, respectively). In the prednisolone group, patients with polyps grade 3 and endoscopic signs of meatal discharge showed significantly less improvement in total NSS, PEFI, and TNPS than patients with grade 1-2 size and negative metal discharge.
Conclusion: In the 12-week treatment evaluation of nasal polyposis, pretreatment with oral steroids had no significant advantage for most nasal symptoms other than earlier relief; however, combined oral and nasal steroid therapy more effectively improved hyposmia, polyps size, and nasal airflow. Polyps size grade 3 and/or endoscopic signs of meatal discharge predisposed to a poorer treatment outcome.
Conflict of interest statement
The authors have no conflicts of interest to declare pertaining to this article
Figures

References
-
- Aouad RK, Chiu AG. State of the art treatment of nasal polyposis. Am J Rhinol Allergy 25:291–298, 2011. - PubMed
-
- Joe SA, Thambi R, Huang J. A systematic review of the use of intranasal steroids in the treatment of chronic rhinosinusitis. Otolaryngol Head Neck Surg 139:340–347, 2008. - PubMed
-
- Badia L, Lund V. Topical corticosteroids in nasal polyposis. Drugs 61:573–578, 2001. - PubMed
-
- Dalziel K, Stein K, Round A, et al. Systematic review of endoscopic sinus surgery for nasal polyps. Health Technol Assess 7:1–159, 2003. - PubMed
-
- Nores JM, Avant P, Bonfils P. Sino-nasal polyposis evaluation of the efficacy of combined local and general corticotherapy in a series of 100 consecutive patients with a 3-year follow-up. Bull Acad Natl Med 186:1643–1656, 2002. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

