High-order chromatin structure and the epigenome in SAHFs
- PMID: 23232545
- PMCID: PMC3585023
- DOI: 10.4161/nucl.23189
High-order chromatin structure and the epigenome in SAHFs
Abstract
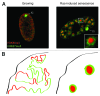
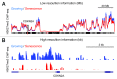
It is almost ten years since senescence associated heterochromatic foci (SAHFs) were first described in human diploid fibroblasts (HDFs). Since then, a number of factors have been identified that affect SAHF formation, including HMGA proteins, structural components of SAHFs. However, the involvement of epigenetic marks in SAHF formation remains unclear. Our recent study, combining microscopy and ChIP-seq approaches, revealed that SAHFs are formed through spatial repositioning of the genome. This occurs according to certain chromatin features that are correlated with, but do not require, the repressive marks histone H3 trimethylated on lysine 9 (H3K9me3) and H3K27me3. These repressive marks are segregated from each other within SAHFs, forming layered high-order chromatin structures (HOCS). During the dynamic change in HOCS as SAHFs form, the linear epigenomic profiles of these repressive marks are highly static. This is in marked contrast to the spreading of repressive marks occurring during embryonic cell differentiation. Thus the layered HOCS of SAHFs is likely achieved mainly through the spatial rearrangement of pre-existing heterochromatin, rather than spreading of heterochromatin. Evidence for the co-association of similar types of chromatin is emerging and SAHFs may provide a unique model system to study the correlation between HOCS and chromatin types, which are readily visible and regulable.
Keywords: SAHF; epigenomics; heterochromatin; histone marks; senescence.
Figures
Comment on
- Chandra T, Kirschner K, Thuret JY, Pope BD, Ryba T, Newman S, Ahmed K, Samarajiwa SA, Salama R, Carroll T, Stark R, Janky R, Narita M, Xue L, Chicas A, Nũnez S, Janknecht R, Hayashi-Takanaka Y, Wilson MD, Marshall A, Odom DT, Babu MM, Bazett-Jones DP, Tavaré S, Edwards PA, Lowe SW, Kimura H, Gilbert DM, Narita M. Independence of repressive histone marks and chromatin compaction during senescent heterochromatic layer formation. Mol Cell. 2012;47:203–14. doi: 10.1016/j.molcel.2012.06.010. doi: 10.1016/j.molcel.2012.06.010
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
