Wired on sugar: the role of the CNS in the regulation of glucose homeostasis
- PMID: 23232606
- PMCID: PMC4231433
- DOI: 10.1038/nrn3409
Wired on sugar: the role of the CNS in the regulation of glucose homeostasis
Abstract
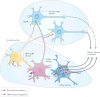
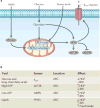
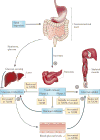
Obesity and type 2 diabetes mellitus (T2DM)--disorders of energy homeostasis and glucose homeostasis, respectively--are tightly linked and the incidences of both conditions are increasing in parallel. The CNS integrates information regarding peripheral nutrient and hormonal changes and processes this information to regulate energy homeostasis. Recent findings indicate that some of the neural circuits and mechanisms underlying energy balance are also essential for the regulation of glucose homeostasis. We propose that disruption of these overlapping pathways links the metabolic disturbances associated with obesity and T2DM. A better understanding of these converging mechanisms may lead to therapeutic strategies that target both T2DM and obesity.
Conflict of interest statement
D.A.S and R.J.S declare competing financial interests: see Web version for details. B.E.G. declares no competing financial interests.
Figures

References
-
- Bernard C. In: Homeostasis: Origins of the Concept, 1973. Langley LL, editor. Dowden, Hutchinson & Ross; Stroudsberg, PA: 1870. pp. 129–151.
-
- Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. - PubMed
-
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature Neurosci. 1998;1:271–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01CA141464/CA/NCI NIH HHS/United States
- DK082480/DK/NIDDK NIH HHS/United States
- U01 CA141464/CA/NCI NIH HHS/United States
- F32 HD068103/HD/NICHD NIH HHS/United States
- RC4 DK090956/DK/NIDDK NIH HHS/United States
- 1F32HD68103/HD/NICHD NIH HHS/United States
- MH069860/MH/NIMH NIH HHS/United States
- R01 DK082480/DK/NIDDK NIH HHS/United States
- DK57900/DK/NIDDK NIH HHS/United States
- DK56863/DK/NIDDK NIH HHS/United States
- P01 DK056863/DK/NIDDK NIH HHS/United States
- R01 DK093848/DK/NIDDK NIH HHS/United States
- R01 MH069860/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources

