The role of arginase I in diabetes-induced retinal vascular dysfunction in mouse and rat models of diabetes
- PMID: 23232640
- PMCID: PMC3565067
- DOI: 10.1007/s00125-012-2789-5
The role of arginase I in diabetes-induced retinal vascular dysfunction in mouse and rat models of diabetes
Abstract
Aims/hypothesis: A reduction in retinal blood flow occurs early in diabetes and is likely to be involved in the development of diabetic retinopathy. We hypothesise that activation of the arginase pathway could have a role in the vascular dysfunction of diabetic retinopathy.
Methods: Experiments were performed using a mouse and rat model of streptozotocin (STZ)-induced diabetes for in vivo and ex vivo analysis of retinal vascular function. For in vivo studies, mice were infused with the endothelial-dependent vasodilator acetylcholine (ACh) or the endothelial-independent vasodilator sodium nitroprusside (SNP), and vasodilation was assessed using a fundus microscope. Ex vivo assays included pressurised vessel myography, western blotting and arginase activity measurements.
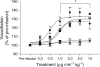
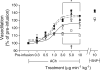
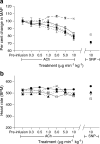
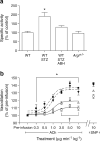
Results: ACh-induced retinal vasodilation was markedly impaired in diabetic mice (40% of control values), whereas SNP-induced dilation was not altered. The diabetes-induced vascular dysfunction was markedly blunted in mice lacking one copy of the gene encoding arginase I and in mice treated with the arginase inhibitor 2(S)-amino-6-boronohexanoic acid. Ex vivo studies performed using pressure myography and central retinal arteries isolated from rats with STZ-induced diabetes showed a similar impairment of endothelial-dependent vasodilation that was partially blunted by pretreatment of the isolated vessels with another arginase inhibitor, (S)-2-boronoethyl-L-cysteine. The diabetes-induced vascular alterations were associated with significant increases in both arginase I protein levels and total arginase activity.
Conclusions/interpretation: These results indicate that, in the mouse and rat model, diabetes-induced increases in arginase I were involved in the diabetes-induced impairment of retinal blood flow by a mechanism involving vascular endothelial cell dysfunction.
Figures




References
-
- Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye (Lond) 2009;23:1496–1508. - PubMed
-
- Clermont AC, Bursell SE. Retinal blood flow in diabetes. Microcirculation. 2007;14:49–61. - PubMed
-
- Nagaoka T, Sato E, Takahashi A, Yokota H, Sogawa K, Yoshida A. Impaired retinal circulation in patients with type 2 diabetes mellitus: retinal laser Doppler velocimetry study. Invest Ophthalmol Vis Sci. 2010;51:6729–6734. - PubMed
-
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

