A randomised controlled trial of bumetanide in the treatment of autism in children
- PMID: 23233021
- PMCID: PMC3565189
- DOI: 10.1038/tp.2012.124
A randomised controlled trial of bumetanide in the treatment of autism in children
Abstract
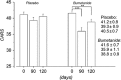
Gamma aminobutyric acid (GABA)-mediated synapses and the oscillations they orchestrate are altered in autism. GABA-acting benzodiazepines exert in some patients with autism paradoxical effects, raising the possibility that like in epilepsies, GABA excites neurons because of elevated intracellular concentrations of chloride. Following a successful pilot study,(1) we have now performed a double-blind clinical trial using the diuretic, chloride-importer antagonist bumetanide that reduces intracellular chloride reinforcing GABAergic inhibition. Sixty children with autism or Asperger syndrome (3-11 years old) received for 3 months placebo or bumetanide (1 mg daily), followed by 1-month wash out. Determination of the severity of autism was made with video films at day 0 (D0) and D90 by blind, independent evaluators. Bumetanide reduced significantly the Childhood Autism Rating Scale (CARS) (D90-D0; P<0.004 treated vs placebo), Clinical Global Impressions (P<0.017 treated vs placebo) and Autism Diagnostic Observation Schedule values when the most severe cases (CARS values above the mean ± s.d.; n=9) were removed (Wilcoxon test: P-value=0.031; Student's t-test: P-value=0.017). Side effects were restricted to an occasional mild hypokalaemia (3.0-3.5 mM l(-1) K(+)) that was treated with supplemental potassium. In a companion study, chronic bumetanide treatment significantly improved accuracy in facial emotional labelling, and increased brain activation in areas involved in social and emotional perception (Hadjikhani et al., submitted). Therefore, bumetanide is a promising novel therapeutic agent to treat autism. Larger trials are warranted to better determine the population best suited for this treatment.
Trial registration: ClinicalTrials.gov NCT01078714.
Figures

References
-
- Lemonnier E, Ben-Ari Y. The diuretic bumetanide decreases autistic behaviour in five infants treated during 3 months with no side effects. Acta Paediatr. 2010;99:1885–1888. - PubMed
-
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, hrens-Barbeau C, Hallet MJ, et al. Neuron number and size in prefrontal cortex of children with autism 1. JAMA. 2011;306:2001–2010. - PubMed
-
- Khan NZ, Gallo LA, Arghir A, Budisteanu B, Budisteanu M, Dobrescu I, et al. Autism and the grand challenges in global mental health. Autism Res. 2012;5:156–159. - PubMed
-
- Lord C, Rutter M, Le CA. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

