Varicella zoster virus infection of highly pure terminally differentiated human neurons
- PMID: 23233078
- PMCID: PMC3568217
- DOI: 10.1007/s13365-012-0142-x
Varicella zoster virus infection of highly pure terminally differentiated human neurons
Abstract
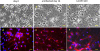
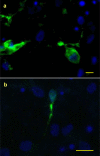
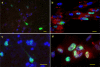
In vitro analyses of varicella zoster virus (VZV) reactivation from latency in human ganglia have been hampered by the inability to isolate virus by explantation or cocultivation techniques. Furthermore, attempts to study interaction of VZV with neurons in experimentally infected ganglion cells in vitro have been impaired by the presence of nonneuronal cells, which become productively infected and destroy the cultures. We have developed an in vitro model of VZV infection in which highly pure (>95 %) terminally differentiated human neurons derived from pluripotent stem cells were infected with VZV. At 2 weeks post-infection, infected neurons appeared healthy compared to VZV-infected human fetal lung fibroblasts (HFLs), which developed a cytopathic effect (CPE) within 1 week. Tissue culture medium from VZV-infected neurons did not produce a CPE in uninfected HFLs and did not contain PCR-amplifiable VZV DNA, but cocultivation of infected neurons with uninfected HFLs did produce a CPE. The nonproductively infected neurons contained multiple regions of the VZV genome, as well as transcripts and proteins corresponding to VZV immediate-early, early, and late genes. No markers of the apoptotic caspase cascade were detected in healthy-appearing VZV-infected neurons. VZV infection of highly pure terminally differentiated human neurons provides a unique in vitro system to study the VZV-neuronal relationship and the potential to investigate mechanisms of VZV reactivation.
Figures




References
-
- Hood C, Cunningham AL, Slobedman B, Boadle RA, Abendroth A. Varicella-zoster virus-infected human sensory neurons are resistant to apoptosis, yet human foreskin fibroblasts are susceptible: evidence for a cell-type-specific apoptotic response. J Virol. 2003;77:12852–12864. doi: 10.1128/JVI.77.23.12852-12864. - PMC - PubMed
-
- Gilden DH, Cohrs RJ, Mahalingam R. Clinical and molecular pathogenesis of varicella virus infection. Viral Immunol. 2003;16:243–258. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

