Immunoconjugates and new molecular targets in hairy cell leukemia
- PMID: 23233649
- PMCID: PMC6290482
- DOI: 10.1182/asheducation-2012.1.660
Immunoconjugates and new molecular targets in hairy cell leukemia
Abstract
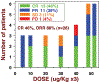
Hairy cell leukemia (HCL) is a B-cell malignancy that in its classic form is exquisitely sensitive to single-agent purine analog therapy, but that is associated in many patients with late relapse and eventual purine analog resistance. Minimal residual disease, which is present in most patients achieving complete remission with purine analogs, retains Ags that are ideal for targeted therapy. Rituximab, which targets CD20, is active as a single agent, particularly if combined with purine analogs. Recombinant immunotoxins targeting either CD25 or CD22 and containing truncated Pseudomonas exotoxin have achieved major responses in relapsed/refractory HCL. Moxetumomab pasudotox in phase 1 testing achieved responses in 86% of such patients (complete in 46%) without dose limiting toxicity and often without MRD. Soluble CD22 has been used for improved detection and monitoring of HCL, particularly the poor-prognosis variant that lacks CD25. Ig rearrangements unique for each HCL patient have been cloned, sequenced, and followed by real-time quantitative PCR using sequence-specific reagents. Analysis of these rearrangements has identified an unmutated IGVH4-34-expressing poor-prognosis variant with immunophenotypic characteristics of either classic or variant HCL. The BRAF V600E mutation, reported in 50% of melanomas, is present in > 85% of HCL cases that are both classic and express rearrangements other than IGVH4-34, making HCL a potential target for specific inhibitors of BRAF V600E. Additional targets are being defined in both classic and variant HCL, which should improve both detection and therapy.
Conflict of interest statement
Disclosure
Conflict-of-interest disclosure: The author is employed by and holds patents with or receives royalties from the National Institutes of Health. Off-label drug use: moxetumomab pasudotox and LMB-2, recombinant immunotoxins targeting CD22 and CD25, respectively.
Figures


References
-
- Bouroncle BA, Wiseman BK, Doan CA. Leukemic reticuloendotheliosis. Blood. 1958;13(7):609–630. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. - PubMed
-
- Matutes E, Oscier D, Montalban C, et al. Splenic marginal zone lymphoma proposals for a revision of diagnostic, staging and therapeutic criteria. Leukemia. 2008;22(3):487–495. - PubMed
-
- Golomb HM, Catovsky D, Golde DW. Hairy cell leukemia: a clinical review based on 71 cases. Ann Intern Med. 1978;89(5 Pt 1):677–683. - PubMed
-
- Habermann TM, Rai K. Historical treatments of in hairy cell leukemia, splenectomy and interferon: past and current uses. Leuk Lymphoma. 2011;52(Suppl 2):18–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

