Genome-wide study of methotrexate clearance replicates SLCO1B1
- PMID: 23233662
- PMCID: PMC3567337
- DOI: 10.1182/blood-2012-08-452839
Genome-wide study of methotrexate clearance replicates SLCO1B1
Abstract
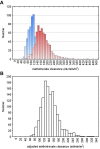
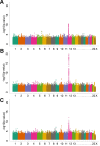
Methotrexate clearance can influence the cure of and toxicity in children with acute lymphoblastic leukemia (ALL). We estimated methotrexate plasma clearance for 1279 patients with ALL treated with methotrexate (24-hour infusion of a 1 g/m2 dose or 4-hour infusion of a 2 g/m2 dose) on the Children’s Oncology Group P9904 and P9905 protocols. Methotrexate clearance was lower in older children (P = 7 x 10(-7)), girls (P = 2.7 x 10(-4)), and those who received a delayed-intensification phase (P = .0022). A genome-wide analysis showed that methotrexate clearance was associated with polymorphisms in the organic anion transporter gene SLCO1B1 (P = 2.1 x 10(-11)). This replicates findings using different schedules of high-dose methotrexate in St Jude ALL treatment protocols; a combined meta-analysis yields a P value of 5.7 x 10(-19) for the association of methotrexate clearance with SLCO1B1 SNP rs4149056. Validation of this variant with 5 different treatment regimens of methotrexate solidifies the robustness of this pharmacogenomic determinant of methotrexate clearance. This study is registered at http://www.clinicaltrials.gov as NCT00005585 and NCT00005596.
Figures


References
-
- Whitehead VM, Shuster JJ, Vuchich MJ, et al. Accumulation of methotrexate and methotrexate polyglutamates in lymphoblasts and treatment outcome in children with B-progenitor-cell acute lymphoblastic leukemia: a Pediatric Oncology Group study. Leukemia. 2005;19(4):533–536. - PubMed
-
- Clarke M, Gaynon P, Hann I, et al. CNS-directed therapy for childhood acute lymphoblastic leukemia: Childhood ALL Collaborative Group overview of 43 randomized trials. J Clin Oncol. 2003;21(9):1798–1809. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

