Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016
- PMID: 23233710
- PMCID: PMC3732010
- DOI: 10.1200/JCO.2012.42.4101
Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016
Abstract
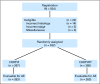
Purpose: Advanced follicular lymphomas (FL) are considered incurable with conventional chemotherapy and there is no consensus on the best treatment approach. Southwest Oncology Group (SWOG) and Cancer and Leukemia Group B compared the safety and efficacy of two immunochemotherapy regimens for FL in a phase III randomized intergroup protocol (SWOG S0016) that enrolled 554 patients with previously untreated, advanced-stage FL between March 1, 2001, and September 15, 2008.
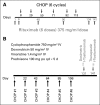
Patients and methods: Patients were eligible for the study if they had advanced-stage (bulky stage II, III, or IV) evaluable FL of any grade (1, 2, or 3) and had not received previous therapy. In one arm of the study, patients received six cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy at 3-week intervals with six doses of rituximab (CHOP-R). In another arm of the study, patients received six cycles of CHOP followed by consolidation with tositumomab/iodine I-131 tositumomab radioimmunotherapy (RIT).
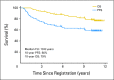
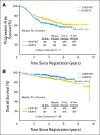
Results: After a median follow-up period of 4.9 years, the 2-year estimate of progression-free survival (PFS) was 76% on the CHOP-R arm and 80% on the CHOP-RIT arm (P = .11). The 2-year estimate of overall survival (OS) was 97% on the CHOP-R arm and 93% on the CHOP-RIT arm (P = .08).
Conclusion: There was no evidence of a significant improvement in PFS comparing CHOP-RIT with CHOP-R. However, PFS and OS were outstanding on both arms of the study. Future studies are needed to determine the potential benefits of combining CHOP-R induction chemotherapy with RIT consolidation and/or extended rituximab maintenance therapy.
Trial registration: ClinicalTrials.gov NCT00006721.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
- 294 doi: 10.1200/JCO.2012.46.2663
References
-
- Press OW. Follicular lymphoma. Williams' Hematology (ed 8) In: Kaushansky K, Lichtman M, Beutler E, et al., editors. New York, NY: McGraw-Hill Medical Publishing Division; 2009. p. 1565.
-
- Fisher RI, LeBlanc M, Press OW, et al. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol . 2005;23:8447–8452. - PubMed
-
- Swenson WT, Wooldridge JE, Lynch CF, et al. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol . 2005;23:5019–5026. - PubMed
-
- Liu Q, Fayad L, Cabanillas F, et al. Improvement of overall and failure-free survival in stage IV follicular lymphoma: 25 years of treatment experience at The University of Texas M.D. Anderson Cancer Center. J Clin Oncol . 2006;24:1582–1589. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
- N01 CA004919/CA/NCI NIH HHS/United States
- U10 CA012644/CA/NCI NIH HHS/United States
- CA35281/CA/NCI NIH HHS/United States
- CA76447/CA/NCI NIH HHS/United States
- CA37981/CA/NCI NIH HHS/United States
- U10 CA022433/CA/NCI NIH HHS/United States
- CA58416/CA/NCI NIH HHS/United States
- U10 CA027057/CA/NCI NIH HHS/United States
- N01 CA035176/CA/NCI NIH HHS/United States
- CA35261/CA/NCI NIH HHS/United States
- U10 CA037981/CA/NCI NIH HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- U10 CA016385/CA/NCI NIH HHS/United States
- CA35128/CA/NCI NIH HHS/United States
- CA22433/CA/NCI NIH HHS/United States
- CA073590/CA/NCI NIH HHS/United States
- U10 CA045560/CA/NCI NIH HHS/United States
- CA12644/CA/NCI NIH HHS/United States
- U10 CA035128/CA/NCI NIH HHS/United States
- CA20319/CA/NCI NIH HHS/United States
- U10 CA076447/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- N01 CA013612/CA/NCI NIH HHS/United States
- U10 CA035192/CA/NCI NIH HHS/United States
- U10 CA045808/CA/NCI NIH HHS/United States
- U10 CA013612/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA058416/CA/NCI NIH HHS/United States
- U10 CA014028/CA/NCI NIH HHS/United States
- N01 CA035119/CA/NCI NIH HHS/United States
- CA45808/CA/NCI NIH HHS/United States
- N01 CA046441/CA/NCI NIH HHS/United States
- R01 CA076287/CA/NCI NIH HHS/United States
- CA14028/CA/NCI NIH HHS/United States
- CA58882/CA/NCI NIH HHS/United States
- CA45377/CA/NCI NIH HHS/United States
- U10 CA035281/CA/NCI NIH HHS/United States
- CA11083/CA/NCI NIH HHS/United States
- CA58861/CA/NCI NIH HHS/United States
- CA35090/CA/NCI NIH HHS/United States
- N01 CA063844/CA/NCI NIH HHS/United States
- CA46282/CA/NCI NIH HHS/United States
- U10 CA035261/CA/NCI NIH HHS/United States
- U10 CA035178/CA/NCI NIH HHS/United States
- CA16385/CA/NCI NIH HHS/United States
- U10 CA045450/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- CA45450/CA/NCI NIH HHS/United States
- CA67663/CA/NCI NIH HHS/United States
- N01 CA035178/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- U10 CA067575/CA/NCI NIH HHS/United States
- CA74811/CA/NCI NIH HHS/United States
- N01 CA027057/CA/NCI NIH HHS/United States
- U10 CA073590/CA/NCI NIH HHS/United States
- U10 CA046441/CA/NCI NIH HHS/United States
- U10 CA045377/CA/NCI NIH HHS/United States
- CA35192/CA/NCI NIH HHS/United States
- U10 CA058882/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- U10 CA074811/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- U10 CA035119/CA/NCI NIH HHS/United States
- U10 CA011083/CA/NCI NIH HHS/United States
- CA52654/CA/NCI NIH HHS/United States
- U10 CA052654/CA/NCI NIH HHS/United States
- CA76429/CA/NCI NIH HHS/United States
- N01 CA067575/CA/NCI NIH HHS/United States
- U10 CA067663/CA/NCI NIH HHS/United States
- U10 CA035176/CA/NCI NIH HHS/United States
- U10 CA035090/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- CA58723/CA/NCI NIH HHS/United States
- U10 CA063844/CA/NCI NIH HHS/United States
- U10 CA058861/CA/NCI NIH HHS/United States
- N01 CA045560/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

