IFN-γ from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection
- PMID: 23233724
- PMCID: PMC3683563
- DOI: 10.4049/jimmunol.1200061
IFN-γ from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection
Abstract
IFN-γ is necessary in both humans and mice for control of Mycobacterium tuberculosis. CD4 T cells are a significant source of IFN-γ during acute infection in mice and are required for control of bacterial growth and host survival. However, several other types of cells can and do produce IFN-γ during the course of the infection. We sought to determine whether IFN-γ from sources other than CD4 T cells was sufficient to control M. tuberculosis infection and whether CD4 T cells had a role in addition to IFN-γ production. To investigate the role of IFN-γ from CD4 T cells, a murine adoptive transfer model was developed in which all cells were capable of producing IFN-γ, with the exception of CD4 T cells. Our data in this system support that CD4 T cells are essential for control of infection, but also that IFN-γ from CD4 T cells is necessary for host survival and optimal long-term control of bacterial burden. In addition, IFN-γ from CD4 T cells was required for a robust CD8 T cell response. IFN-γ from T cells inhibited intracellular replication of M. tuberculosis in macrophages, suggesting IFN-γ may be necessary for intracellular bactericidal activity. Thus, although CD4 T cells play additional roles in the control of M. tuberculosis infection, IFN-γ is a major function by which these cells participate in resistance to tuberculosis.
Figures

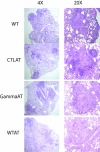
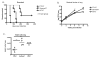




Similar articles
-
CD4+ T cell-dependent IFN-γ production by CD8+ effector T cells in Mycobacterium tuberculosis infection.J Immunol. 2012 Sep 1;189(5):2530-6. doi: 10.4049/jimmunol.1200994. Epub 2012 Jul 25. J Immunol. 2012. PMID: 22837486 Free PMC article.
-
CD4+ T cells mediate IFN-gamma-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo.J Immunol. 2003 Nov 1;171(9):4689-99. doi: 10.4049/jimmunol.171.9.4689. J Immunol. 2003. PMID: 14568944
-
CD4 T Cell-Derived IFN-γ Plays a Minimal Role in Control of Pulmonary Mycobacterium tuberculosis Infection and Must Be Actively Repressed by PD-1 to Prevent Lethal Disease.PLoS Pathog. 2016 May 31;12(5):e1005667. doi: 10.1371/journal.ppat.1005667. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27244558 Free PMC article.
-
[Protective immunity against Mycobacterium tuberculosis].Kekkaku. 2006 Nov;81(11):687-91. Kekkaku. 2006. PMID: 17154048 Review. Japanese.
-
CD4 T-cell immunotherapy for chronic viral infections and cancer.Immunotherapy. 2013 Sep;5(9):975-87. doi: 10.2217/imt.13.91. Immunotherapy. 2013. PMID: 23998732 Free PMC article. Review.
Cited by
-
De novo histidine biosynthesis protects Mycobacterium tuberculosis from host IFN-γ mediated histidine starvation.Commun Biol. 2021 Mar 25;4(1):410. doi: 10.1038/s42003-021-01926-4. Commun Biol. 2021. PMID: 33767335 Free PMC article.
-
Intranasal Immunization With Nanoparticles Containing an Orientia tsutsugamushi Protein Vaccine Candidate and a Polysorbitol Transporter Adjuvant Enhances Both Humoral and Cellular Immune Responses.Immune Netw. 2023 Dec 15;23(6):e47. doi: 10.4110/in.2023.23.e47. eCollection 2023 Dec. Immune Netw. 2023. PMID: 38188601 Free PMC article.
-
M. tuberculosis CRISPR/Cas proteins are secreted virulence factors that trigger cellular immune responses.Virulence. 2021 Dec;12(1):3032-3044. doi: 10.1080/21505594.2021.2007621. Virulence. 2021. PMID: 34886764 Free PMC article.
-
Vaccines for Leprosy and Tuberculosis: Opportunities for Shared Research, Development, and Application.Front Immunol. 2018 Feb 26;9:308. doi: 10.3389/fimmu.2018.00308. eCollection 2018. Front Immunol. 2018. PMID: 29535713 Free PMC article. Review.
-
T-cell autophagy deficiency increases mortality and suppresses immune responses after sepsis.PLoS One. 2014 Jul 16;9(7):e102066. doi: 10.1371/journal.pone.0102066. eCollection 2014. PLoS One. 2014. PMID: 25029098 Free PMC article.
References
-
- Saito S, Nakano M. Nitric oxide production by peritoneal macrophages of Mycobacterium bovis BCG-infected or non-infected mice: regulatory role of T lymphocytes and cytokines. J Leukoc Biol. 1996;59(6):908–15. - PubMed
-
- Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

