Galantamine versus donepezil in Chinese patients with Alzheimer's disease: results from a randomized, double-blind study
- PMID: 23233806
- PMCID: PMC3518285
- DOI: 10.2147/NDT.S38747
Galantamine versus donepezil in Chinese patients with Alzheimer's disease: results from a randomized, double-blind study
Abstract
Background: Acetylcholinesterase inhibitors are considered standard of care for Alzheimer's disease in many countries. Galantamine is an acetylcholinesterase inhibitor that may also act via allosteric modulation of nicotinic acetylcholine receptors. Therefore, it may provide benefits compared with other acetylcholinesterase inhibitors. The present study compared galantamine (n = 116) with donepezil (n = 117) in a double-blind trial at nine hospitals in China.
Methods: After washout of any previous acetylcholinesterase inhibitors, subjects with mild to moderate Alzheimer's disease received galantamine or donepezil for 16 weeks.
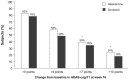
Results: Alzheimer's Disease Assessment Scale - cognitive subscale (ADAS-cog/11) scores improved significantly from baseline in both treatment arms, with a significant difference in favor of galantamine on the "language" functional area (P = 0.035). Significantly more galantamine-treated patients responded to treatment (defined as a reduction in ADAS-cog/11 score of >4, >7, or >10 points; all P < 0.05), and had an ADAS-cog/11 score < 20 at end point (P = 0.015). Both treatments were well tolerated, although fewer galantamine-treated patients experienced gastrointestinal adverse events compared with donepezil (30% versus 48%).
Conclusion: Cognitive function improved significantly in subjects with mild to moderate Alzheimer's disease treated with galantamine or donepezil, and both treatments were generally well tolerated. Significant benefits for galantamine over donepezil were observed for language and response to treatment.
Keywords: Alzheimer’s disease; Chinese; donepezil; galantamine; randomized controlled trial.
Figures

References
-
- National Institute for Health and Clinical Excellence. Donepezil, Galantamine, Rivastigmine (Review) and Memantine for the Treatment of Alzheimer’s Disease (Amended) London, UK: National Institute for Health and Clinical Excellence; 2007.
-
- Deutsche Gesellschaft für Psychiatrie Psychotherapie und Nervenheilkunde, Deutsche Gesellschaft für Neurologie. S3-Leitlinie “Demenzen”. 2009. [Accessed January 15, 2011]. Available from: http://www.dgn.org/images/stories/dgn/leitlinien/ll_demenz/ll-demenz-kur....
-
- Socialstyrelsen. Västerås: Edita Västra Aros; 2010. Nationella riktlinjer för vård och omsorg vid demenssjukdom 2010 – stöd för styrning och ledning.
-
- American Psychiatric Association. Practice Guideline for the Treatment of Patients with Alzheimer’s Disease and Other Dementias. 2nd ed. Arlington, VA: American Psychiatric Publishing Inc; 2007. - PubMed
-
- Samochocki M, Hoffle A, Fehrenbacher A, et al. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;305:1024–1036. - PubMed
LinkOut - more resources
Full Text Sources

