Chiral-anion-dependent inversion of diastereo- and enantioselectivity in carbonyl crotylation via ruthenium-catalyzed butadiene hydrohydroxyalkylation
- PMID: 23234459
- PMCID: PMC3531795
- DOI: 10.1021/ja311208a
Chiral-anion-dependent inversion of diastereo- and enantioselectivity in carbonyl crotylation via ruthenium-catalyzed butadiene hydrohydroxyalkylation
Abstract
The ruthenium catalyst generated in situ from H(2)Ru(CO)(PPh(3))(3), (S)-SEGPHOS, and a TADDOL-derived phosphoric acid promotes butadiene hydrohydroxyalkylation to form enantiomerically enriched products. Notably, the observed diastereo- and enantioselectivity is the opposite of that observed using BINOL-derived phosphate counterions in combination with (S)-SEGPHOS, the same enantiomer of the chiral ligand. Match/mismatch effects between the chiral ligand and the chiral TADDOL-phosphate counterion are described. For the first time, single-crystal X-ray diffraction data for a ruthenium complex modified by a chiral phosphate counterion are reported.
Figures



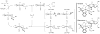
References
-
-
For recent reviews of C-C bond forming hydrogenation and transfer hydrogenation, see: Bower JF, Krische MJ. Top Organomet Chem. 2011;43:107.Hassan A, Krische MJ. Org Proc Res Devel. 2011;15:1236.Moran J, Krische MJ. Pure Appl Chem. 2012;84:1729.
-
-
-
For iridium catalyzed carbonyl crotylation from the alcohol oxidation level employing α-methyl allyl acetate as the crotyl donor, see: Kim IS, Han SB, Krische MJ. J Am Chem Soc. 2009;131:2514.Gao X, Townsend IA, Krische MJ. J Org Chem. 2011;76:2350.Gao X, Han H, Krische MJ. J Am Chem Soc. 2011;133:12795.
-
-
-
For selected reviews on enantioselective carbonyl allylation and crotylation, see: Yamamoto Y, Asao N. Chem Rev. 1993;93:2207.Ramachandran PV. Aldrichim Acta. 2002;35:23.Kennedy JWJ, Hall DG. Angew Chem, Int Ed. 2003;42:4732.Denmark SE, Fu J. Chem Rev. 2003;103:2763.Yu CM, Youn J, Jung HK. Bull Korean Chem Soc. 2006;27:463.Marek I, Sklute G. Chem Commun. 2007:1683.Hall DG. Synlett. 2007:1644.Li J, Menche D. Synthesis. 2009:2293.Leighton JL. Aldrichim Acta. 2010;43:3.Yus M, González-Gómez JC, Foubelo F. Chem Rev. 2011;111:7774.
-
-
-
For iridium and ruthenium catalyzed hydrohydroxyalkylations of butadiene to form products of crotylation, see: Shibahara F, Bower JF, Krische MJ. J Am Chem Soc. 2008;130:6338.Zbieg JR, Fukuzumi T, Krische MJ. Adv Synth Catal. 2010;352:2416.Zbieg JR, Moran J, Krische MJ. J Am Chem Soc. 2011;133:10582.Zbieg JR, Yamaguchi E, McInturff EL, Krische MJ. Science. 2012;336:324.
-
-
-
For selected reviews on chiral phosphate counterions in metal catalysis, see: Shao Z, Zhang H. Chem Soc Rev. 2009;38:2745.Zhong C, Shi X. Eur J Org Chem. 2010:2999.Rueping M, Koenigs RM, Atodiresei I. Chem Eur J. 2010;16:9350.Phipps RJ, Hamilton GL, Toste FD. Nat Chem. 2012;4:603.Parra A, Reboredo S, Martin Castro AM, Aleman J. Org Biomol Chem. 2012;10:5001.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

