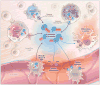
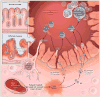
Purinergic signaling during inflammation
- PMID: 23234515
- PMCID: PMC3675791
- DOI: 10.1056/NEJMra1205750
Purinergic signaling during inflammation
Figures


Comment in
-
Purinergic signaling during inflammation.N Engl J Med. 2013 Mar 28;368(13):1260. doi: 10.1056/NEJMc1300259. N Engl J Med. 2013. PMID: 23534573 No abstract available.
-
Purinergic signaling during inflammation.N Engl J Med. 2013 Mar 28;368(13):1260. doi: 10.1056/NEJMc1300259. N Engl J Med. 2013. PMID: 23534574 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
