Efficacy and tolerability of a new formulation of artesunate-mefloquine for the treatment of uncomplicated malaria in adult in Senegal: open randomized trial
- PMID: 23234606
- PMCID: PMC3554515
- DOI: 10.1186/1475-2875-11-416
Efficacy and tolerability of a new formulation of artesunate-mefloquine for the treatment of uncomplicated malaria in adult in Senegal: open randomized trial
Abstract
Background: Prompt treatment of malaria attacks with arteminisin-based combination therapy (ACT) is an essential tool for malaria control. A new co-blister tablet of artesunate-mefloquine (AM) with 25 mg/kg mefloquine has been developed for the management of uncomplicated malaria attacks. This non-inferiority randomized trial, was conducted to evaluate the efficacy and safety of the new formulation of AM in comparison to artemether-lumefantrine (AL) for the treatment of acute uncomplicated Plasmodium falciparum malaria in adults in Senegal.
Methods: The study was carried out from September to December 2010 in two health centres in Senegal. The study end points included (i) PCR corrected adequate clinical and parasitological response (ACPR) at day 28, (ii) ACPR at days 42 and 63, (iii) parasites and fever clearance time, (iv) incidence of adverse events and patients biological profile at day 7 using the WHO 2003 protocol for anti-malarial drug evaluation.
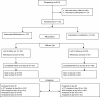
Results: Overall, 310 patients were randomized to receive either AM (n = 157) or AL (n = 153). PCR corrected ACPR at day 28 was at 95.5% in the AM arm while that in the AL arm was at 96.7% (p = 0.83). Therapeutic efficacy was at 98.5% in the AM arm versus 98.2% in the AL group at day 42 (p = 1). At day 63, ACPR in the AM and AL arms was at 98.2% and 97.7%, respectively (p = 0.32). The two treatments were well tolerated with similar biological profile at day 7. However, dizziness was more frequent in the AM arm.
Conclusion: Artesunate-mefloquine (25 mg/Kg mefloquine) is efficacious and well-tolerated for the treatment of uncomplicated P. falciparum malaria in adult patients.
Figures
References
-
- WHO. World Malaria Report. Geneva: World Health Organization; 2011.
-
- WHO. Guidelines for the treatment of malaria. Second. Geneva: WHO Press; 2010.
-
- WHO. Guidelines for the treatment of malaria. Geneva: World Health Organization; 2006.
-
- WHO. Antimalarial drug combination therapy: Report of a WHO Technical Consultation. Geneva: WHO Press; 2001.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources