Organic solar cells: understanding the role of Förster resonance energy transfer
- PMID: 23235328
- PMCID: PMC3546737
- DOI: 10.3390/ijms131217019
Organic solar cells: understanding the role of Förster resonance energy transfer
Abstract
Organic solar cells have the potential to become a low-cost sustainable energy source. Understanding the photoconversion mechanism is key to the design of efficient organic solar cells. In this review, we discuss the processes involved in the photo-electron conversion mechanism, which may be subdivided into exciton harvesting, exciton transport, exciton dissociation, charge transport and extraction stages. In particular, we focus on the role of energy transfer as described by F¨orster resonance energy transfer (FRET) theory in the photoconversion mechanism. FRET plays a major role in exciton transport, harvesting and dissociation. The spectral absorption range of organic solar cells may be extended using sensitizers that efficiently transfer absorbed energy to the photoactive materials. The limitations of F¨orster theory to accurately calculate energy transfer rates are discussed. Energy transfer is the first step of an efficient two-step exciton dissociation process and may also be used to preferentially transport excitons to the heterointerface, where efficient exciton dissociation may occur. However, FRET also competes with charge transfer at the heterointerface turning it in a potential loss mechanism. An energy cascade comprising both energy transfer and charge transfer may aid in separating charges and is briefly discussed. Considering the extent to which the photo-electron conversion efficiency is governed by energy transfer, optimisation of this process offers the prospect of improved organic photovoltaic performance and thus aids in realising the potential of organic solar cells.
Figures






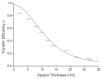
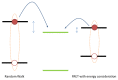
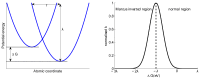

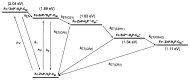
References
-
- Mishra A., Bäuerle P. Small molecule organic semiconductors on the move: Promises for future solar energy technology. Angew. Chem. Int. Ed. 2012;51:2020–2067. - PubMed
-
- Green M.A., Emery K., Hishikawa Y., Warta W., Dunlop E.D. Solar cell efficiency tables (version 39) Prog. Photovoltaics Res. Appl. 2012;20:12–20.
-
- National Renewable Energy Laboratory. Best Research-Cell Efficiencies. [accessed on 11 December 2012]. Available online: http://www.nrel.gov/ncpv/images/efficiencychart.jpg.
-
- Li Y. Molecular design of photovoltaic materials for polymer solar cells: Toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 2012;45:723–733. - PubMed
-
- Li G., Zhu R., Yang Y. Polymer solar cells. Nat. Photonics. 2012;6:153–161.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

