Heterologous downregulation of vasopressin type 2 receptor is induced by transferrin
- PMID: 23235478
- PMCID: PMC3602717
- DOI: 10.1152/ajprenal.00438.2011
Heterologous downregulation of vasopressin type 2 receptor is induced by transferrin
Abstract
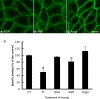
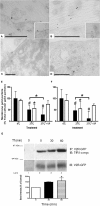
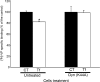
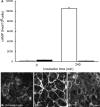
Vasopressin (VP) binds to the vasopressin type 2 receptor (V2R) to trigger physiological effects including body fluid homeostasis and blood pressure regulation. Signaling is terminated by receptor downregulation involving clathrin-mediated endocytosis and V2R degradation. We report here that both native and epitope-tagged V2R are internalized from the plasma membrane of LLC-PK1 kidney epithelial cells in the presence of another ligand, transferrin (Tf). The presence of iron-saturated Tf (holo-Tf; 4 h) reduced V2R binding sites at the cell surface by up to 33% while iron-free (apo-Tf) had no effect. However, no change in green fluorescent protein-tagged V2R distribution was observed in the presence of bovine serum albumin, atrial natriuretic peptide, or ANG II. Conversely, holo-Tf did not induce the internalization of another G protein-coupled receptor, the parathyroid hormone receptor. In contrast to the effect of VP, Tf did not increase intracellular cAMP or modify aquaporin-2 distribution in these cells, although addition of VP and Tf together augmented VP-induced V2R internalization. Tf receptor coimmunoprecipitated with V2R, suggesting that they interact closely, which may explain the additive effect of VP and Tf on V2R endocytosis. Furthermore, Tf-induced V2R internalization was abolished in cells expressing a dominant negative dynamin (K44A) mutant, indicating the involvement of clathrin-coated pits. We conclude that Tf can induce heterologous downregulation of the V2R and this might desensitize VP target cells without activating downstream V2R signaling events. It also provides new insights into urine-concentrating defects observed in rat models of hemochromatosis.
Figures



Similar articles
-
Aquaporin 2 (AQP2) and vasopressin type 2 receptor (V2R) endocytosis in kidney epithelial cells: AQP2 is located in 'endocytosis-resistant' membrane domains after vasopressin treatment.Biol Cell. 2006 Apr;98(4):215-32. doi: 10.1042/BC20040054. Biol Cell. 2006. PMID: 16563128
-
Visualizing microtubule-dependent vasopressin type 2 receptor trafficking using a new high-affinity fluorescent vasopressin ligand.Endocrinology. 2011 Oct;152(10):3893-904. doi: 10.1210/en.2011-1049. Epub 2011 Aug 9. Endocrinology. 2011. PMID: 21828182 Free PMC article.
-
Downregulation of the vasopressin type 2 receptor after vasopressin-induced internalization: involvement of a lysosomal degradation pathway.Am J Physiol Cell Physiol. 2005 Jun;288(6):C1390-401. doi: 10.1152/ajpcell.00353.2004. Epub 2005 Jan 26. Am J Physiol Cell Physiol. 2005. PMID: 15677378
-
The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors.Am J Physiol Renal Physiol. 2014 May 1;306(9):F931-40. doi: 10.1152/ajprenal.00604.2013. Epub 2014 Mar 5. Am J Physiol Renal Physiol. 2014. PMID: 24598801 Review.
-
Vasopressin receptor-mediated endocytosis: current view.Am J Physiol. 1991 Jul;261(1 Pt 2):F1-13. doi: 10.1152/ajprenal.1991.261.1.F1. Am J Physiol. 1991. PMID: 1650143 Review.
Cited by
-
EGF Receptor Inhibition by Erlotinib Increases Aquaporin 2-Mediated Renal Water Reabsorption.J Am Soc Nephrol. 2016 Oct;27(10):3105-3116. doi: 10.1681/ASN.2015080903. Epub 2016 Mar 9. J Am Soc Nephrol. 2016. PMID: 27694161 Free PMC article.
-
Actin-related protein 2/3 complex plays a critical role in the aquaporin-2 exocytotic pathway.Am J Physiol Renal Physiol. 2021 Aug 1;321(2):F179-F194. doi: 10.1152/ajprenal.00015.2021. Epub 2021 Jun 28. Am J Physiol Renal Physiol. 2021. PMID: 34180716 Free PMC article.
-
Large G protein α-subunit XLαs limits clathrin-mediated endocytosis and regulates tissue iron levels in vivo.Proc Natl Acad Sci U S A. 2017 Nov 7;114(45):E9559-E9568. doi: 10.1073/pnas.1712670114. Epub 2017 Oct 23. Proc Natl Acad Sci U S A. 2017. PMID: 29078380 Free PMC article.
References
-
- Bachvarov DR, Houle S, Bachvarova M, Bouthillier J, Adam A, Marceau F. Bradykinin B(2) receptor endocytosis, recycling, and down-regulation assessed using green fluorescent protein conjugates. J Pharmacol Exp Ther 297: 19–26, 2001 - PubMed
-
- Baldwin GS. Comparison of transferrin sequences from different species. Comp Biochem Physiol B 106: 203–218, 1993 - PubMed
-
- Bider MD, Spiess M. Ligand-induced endocytosis of the asialoglycoprotein receptor: evidence for heterogeneity in subunit oligomerization. FEBS Lett 434: 37–41, 1998 - PubMed
-
- Birnbaumer M. Vasopressin receptor mutations and nephrogenic diabetes insipidus. Arch Med Res 30: 465–474, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

