Calciomics: integrative studies of Ca2+-binding proteins and their interactomes in biological systems
- PMID: 23235533
- PMCID: PMC3614492
- DOI: 10.1039/c2mt20009k
Calciomics: integrative studies of Ca2+-binding proteins and their interactomes in biological systems
Abstract
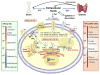
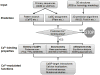
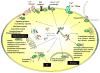
Calcium ion (Ca(2+)), the fifth most common chemical element in the earth's crust, represents the most abundant mineral in the human body. By binding to a myriad of proteins distributed in different cellular organelles, Ca(2+) impacts nearly every aspect of cellular life. In prokaryotes, Ca(2+) plays an important role in bacterial movement, chemotaxis, survival reactions and sporulation. In eukaryotes, Ca(2+) has been chosen through evolution to function as a universal and versatile intracellular signal. Viruses, as obligate intracellular parasites, also develop smart strategies to manipulate the host Ca(2+) signaling machinery to benefit their own life cycles. This review focuses on recent advances in applying both bioinformatic and experimental approaches to predict and validate Ca(2+)-binding proteins and their interactomes in biological systems on a genome-wide scale (termed "calciomics"). Calmodulin is used as an example of Ca(2+)-binding protein (CaBP) to demonstrate the role of CaBPs on the regulation of biological functions. This review is anticipated to rekindle interest in investigating Ca(2+)-binding proteins and Ca(2+)-modulated functions at the systems level in the post-genomic era.
Figures


References
-
- Ross MD. The influence of gravity on structure and function of animals. Adv Spce Res. 1984;4:305–14. - PubMed
-
- Stini WA. Calcium homeostasis and human evolution. Coll Antropol. 1998;22:411–25. - PubMed
- Case RM, Eisner D, Gurney A, Jones O, Muallem S, Verkhratsky A. Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signalling system. Cell Calcium. 2007;42:345–50. doi: 10.1016/j.ceca.2007.05.001. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

