Ursodeoxycholic acid for primary biliary cirrhosis
- PMID: 23235576
- PMCID: PMC7045744
- DOI: 10.1002/14651858.CD000551.pub3
Ursodeoxycholic acid for primary biliary cirrhosis
Abstract
Background: Ursodeoxycholic acid is administered to patients with primary biliary cirrhosis, a chronic progressive inflammatory autoimmune-mediated liver disease with unknown aetiology. Despite its controversial effects, the U.S. Food and Drug Administration has approved its usage for primary biliary cirrhosis.
Objectives: To assess the beneficial and harmful effects of ursodeoxycholic acid in patients with primary biliary cirrhosis.
Search methods: We searched for eligible randomised trials in The Cochrane Hepato-Biliary Group Controlled Trials Register, The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, LILACS, Clinicaltrials.gov, and the WHO International Clinical Trials Registry Platform. The literature search was performed until January 2012.
Selection criteria: Randomised clinical trials assessing the beneficial and harmful effects of ursodeoxycholic acid versus placebo or 'no intervention' in patients with primary biliary cirrhosis.
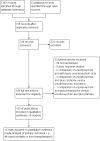
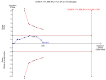
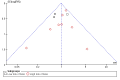
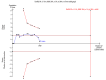
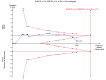
Data collection and analysis: Two authors independently extracted data. Continuous data were analysed using mean difference (MD) and standardised mean difference (SMD). Dichotomous data were analysed using risk ratio (RR). Meta-analyses were conducted using both a random-effects model and a fixed-effect model, with 95% confidence intervals (CI). Random-effects model meta-regression was used to assess the effects of covariates across the trials. Trial sequential analysis was used to assess risk of random errors (play of chance). Risks of bias (systematic error) in the included trials were assessed according to Cochrane methodology bias domains.
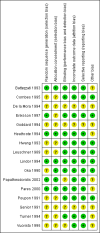
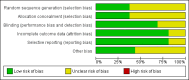
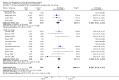
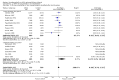
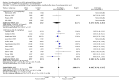
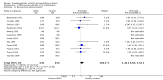
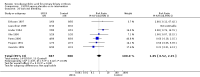
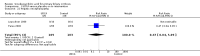
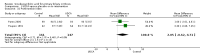
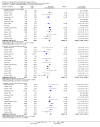
Main results: Sixteen randomised clinical trials with 1447 patients with primary biliary cirrhosis were included. One trial had low risk of bias, and the remaining fifteen had high risk of bias. Fourteen trials compared ursodeoxycholic acid with placebo and two trials compared ursodeoxycholic acid with 'no intervention'. The percentage of patients with advanced primary biliary cirrhosis at baseline varied from 15% to 83%, with a median of 51%. The duration of the trials varied from 3 to 92 months, with a median of 24 months. The results showed no significant difference in effect between ursodeoxycholic acid and placebo or 'no intervention' on all-cause mortality (45/699 (6.4%) versus 46/692 (6.6%); RR 0.97, 95% CI 0.67 to 1.42, I² = 0%; 14 trials); on all-cause mortality or liver transplantation (86/713 (12.1%) versus 89/706 (12.6%); RR 0.96, 95% CI 0.74 to 1.25, I² = 15%; 15 trials); on serious adverse events (94/695 (13.5%) versus 107/687 (15.6%); RR 0.87, 95% CI 0.68 to 1.12, I² = 23%; 14 trials); or on non-serious adverse events (27/643 (4.2%) versus 18/634 (2.8%); RR 1.46, 95% CI 0.83 to 2.56, I² = 0%; 12 trials). The random-effects model meta-regression showed that the risk of bias of the trials, disease severity of patients at entry, ursodeoxycholic acid dosage, and trial duration were not significantly associated with the intervention effects on all-cause mortality, or on all-cause mortality or liver transplantation. Ursodeoxycholic acid did not influence the number of patients with pruritus (168/321 (52.3%) versus 166/309 (53.7%); RR 0.96, 95% CI 0.84 to 1.09, I² = 0%; 6 trials) or with fatigue (170/252 (64.9%) versus 174/244 (71.3%); RR 0.90, 95% CI 0.81 to 1.00, I² = 62%; 4 trials). Two trials reported the number of patients with jaundice and showed a significant effect of ursodeoxycholic acid versus placebo or no intervention in a fixed-effect meta-analysis (5/99 (5.1%) versus 15/99 (15.2%); RR 0.35, 95% CI 0.14 to 0.90, I² = 51%; 2 trials). The result was not supported by the random-effects meta-analysis (RR 0.56, 95% CI 0.06 to 4.95). Portal pressure, varices, bleeding varices, ascites, and hepatic encephalopathy were not significantly affected by ursodeoxycholic acid. Ursodeoxycholic acid significantly decreased serum bilirubin concentration (MD -8.69 µmol/l, 95% CI -13.90 to -3.48, I² = 0%; 881 patients; 9 trials) and activity of serum alkaline phosphatases (MD -257.09 U/L, 95% CI -306.25 to -207.92, I² = 0%; 754 patients, 9 trials) compared with placebo or no intervention. These results were supported by trial sequential analysis. Ursodeoxycholic acid also seemed to improve serum levels of gamma-glutamyltransferase, aminotransferases, total cholesterol, and plasma immunoglobulin M concentration. Ursodeoxycholic acid seemed to have a beneficial effect on worsening of histological stage (random; 66/281 (23.5%) versus 103/270 (38.2%); RR 0.62, 95% CI 0.44 to 0.88, I² = 35%; 7 trials).
Authors' conclusions: This systematic review did not demonstrate any significant benefits of ursodeoxycholic acid on all-cause mortality, all-cause mortality or liver transplantation, pruritus, or fatigue in patients with primary biliary cirrhosis. Ursodeoxycholic acid seemed to have a beneficial effect on liver biochemistry measures and on histological progression compared with the control group. All but one of the included trials had high risk of bias, and there are risks of outcome reporting bias and risks of random errors as well. Randomised trials with low risk of bias and low risks of random errors examining the effects of ursodeoxycholic acid for primary biliary cirrhosis are needed.
Conflict of interest statement
None known.
Figures


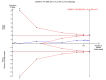

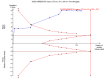




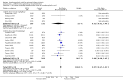
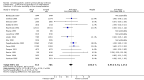

























Update of
-
Ursodeoxycholic acid for primary biliary cirrhosis.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD000551. doi: 10.1002/14651858.CD000551.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2012 Dec 12;12:CD000551. doi: 10.1002/14651858.CD000551.pub3. PMID: 18677775 Updated.
References
References to studies included in this review
Battezzati 1993 {published data only}
-
- Battezzati PM, Podda M, Bianchi FB, Naccarato R, Orlandi F, Surrenti C, et al. Ursodeoxycholic acid for symptomatic primary biliary cirrhosis. Preliminary analysis of a double‐blind multicenter trial. Italian Multicenter Group for the Study of UDCA in PBC. Journal of Hepatology 1993;17:332‐8. - PubMed
-
- Italian Multicenter Project for UDCA Treatment in PBC. Ursodeoxycholic acid (UDCA) for symptomatic primary biliary cirrhosis (PBC): a double‐blind multicenter trial (EASL abstract). Journal of Hepatology 1989;9(Suppl 1):87.
-
- Podda M, Almasio P, Battezzati PM, Crosignani A, and Italian Multicenter Group for the Study of UDCA in PBC. Long‐term effect of the administration of ursodeoxycholic acid alone or with colchicine in patients with primary biliary cirrhosis: a double‐blind multicenter study. Bile Acids and the Hepatobiliary System. From Basic Science to Clinical Practice. Falk Symposium 68. 1993:310‐5.
-
- Podda M, Battezzati PM, Crosignani A, Bianchi FB, Fusconi M, Chiaramonte M, et al. Urodeoxycholic acid (UDCA) for symptomatic primary biliary cirrhosis (PBC): a double‐blind multicenter trial [AASLD abstract]. Hepatology 1989;10:639.
Combes 1995 {published data only}
-
- Combes B, Carithers RL Jr, Maddrey WC, Lin D, McDonald MF, Wheeler DE, et al. A randomised, double‐blind, placebo‐controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 1995;22:759‐66. - PubMed
-
- Combes B, Carithers RL Jr, McDonald MF, Maddrey WC, Munoz SJ, Boyer JL, et al. Ursodeoxycholic acid therapy in patients with primary biliary cirrhosis [AASLD abstract]. Hepatology 1991;14:91A.
-
- Combes B, Carithers RL, Maddrey WC, Munoz SJ, McDonald MF, Garcia‐Tsao G, et al. A randomised, double‐blind, placebo‐controlled trial of ursodeoxycholic acid (UDCA) in primary biliary cirrhosis (AASLD Abstract). Hepatology 1993;18:175A. - PubMed
De la Mora 1994 {published data only}
-
- Mora G, Bobadilla J, Romero P, Rodríguez‐Leal G, Morán S, Kershenobich D, et al. Does treatment with ursodeoxycholic acid (UDCA) really diminish cholesterol serum levels in primary biliary cirrhosis (PBC)? [EASL abstract]. Hepatology 1994;19:57I.
Eriksson 1997 {published and unpublished data}
-
- Eriksson LS, Olsson R, Glauman H, Prytz H, Befrits H, Lindgren S, et al. Ursodeoxycholic acid (UDCA) in patients with primary biliary cirrhosis (PBC) Results of a two‐year randomised placebo‐controlled study (abstract). Scandinavian Journal of Gastroenterology 1995;30(Suppl 209):35. - PubMed
-
- Eriksson LS, Olsson R, Glauman H, Prytz H, Befrits R, Ryden BO, et al. Ursodeoxycholic acid treatment in patients with primary biliary cirrhosis. A Swedish multicenter, double‐blind, randomised controlled study. Scandinavian Journal of Gastroenterology 1997;32:179‐86. - PubMed
Goddard 1994 {published data only}
-
- Goddard CJR, Hunt L, Smith A, Fallowfield G, Rowan B, Warnes TW. A trial of ursodeoxycholic acid (UDCA) and colchicine in primary biliary cirrhosis (PBC) (AASLD abstract). Hepatology 1994;20:151A.
-
- Goddard CJR, Smith A, Hunt L, Halder T, Hillier V, Rowan B, et al. Surrogate markers of response in a trial of ursodeoxycholic acid (UDCA) and colchicine in primary biliary cirrhosis (PBC). Gut 1995;36(Suppl 1):A30.
Heathcote 1994 {published data only}
-
- Ghent CN, Cauch‐Dudek K, Heathcote EJ, and the Canadian PBC Trial Group. Ursodeoxycholic acid therapy effects on pruritus and fatigue in primary biliary cirrhosis. Hepatology 1997;26:438 A.
-
- Heathcote EJ, Cauch DK, Walker V, Bailey RJ, Blendis LM, Ghent CN, et al. The Canadian Multicenter Double‐blind Randomized Controlled Trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 1994;19:1149‐56. - PubMed
-
- Heathcote EJL, Cauch K, Walker V, Bailey RJ, Blendis LM, Ghent CN, et al. A double blind randomised controlled multi‐centre trial of ursodeoxycholic acid (UDCA) in primary biliary cirrhosis (PBC): results from a blinded interim analysis. XII International Bile Acid Meeting. Bile Acids and the Hepatobiliary System. From Basic Science to Clinical Practice. Basel. Falk Symposium No. 68. 1992:45.
-
- Heathcote EJL, Cauch K, Walker V, Bailey RJ, Blendis LM, Ghent CN, et al. The Canadian multi‐centre double blind randomised controlled trial of ursodeoxycholic acid in primary biliary cirrhosis [AASLD abstract]. Hepatology 1992;16:91A. - PubMed
-
- Heathcote EJL, Cauch K, Walker V, Blendis LM, Ghent CN, Pappas SC, et al. A four‐year follow‐up study of ursodeoxycholic acid therapy for primary biliary cirrhosis. Gastroenterology 1993;104:A914.
Hwang 1993 {published and unpublished data}
-
- Hwang SJ, Chan CY, Lee SD, Wu JC, Tsay SH, Lo KJ. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a short‐term, randomised, double‐blind controlled, cross‐over study with long‐term follow up. Journal of Gastroenterology and Hepatology 1993;8:217‐23. - PubMed
Leuschner 1989 {published and unpublished data}
-
- Güldütuna S, Leuschner U, Imhof M, Zimmer G. Treatment of chronic active hepatitis and primary biliary cirrhosis with ursodeoxycholic acid. Zeitschrift fur Gastroenterologie 1992;30 Suppl 1:49‐54. - PubMed
-
- Leuschner M, Güldütuna S, Imhof M, Bhati S, You T, Leuschner U. Ursodeoxycholic acid therapy in primary biliary cirrhosis. Bile acids and the hepatobiliary system. From Basic Science to Clinical Practice. Falk Symposium 68. 1993:299‐302.
-
- Leuschner U, Fischer H, Güldütuna S, Kurtz W, Gatzen M, Hellstern A, et al. Does ursodeoxycholic acid (UDCA) influence cell membrane architecture in patients with primary biliary cirrhosis (PBC)?. Gastroenterology 1989;96:A621. - PubMed
-
- Leuschner U, Fischer H, Hübner K. [Ursodeoxycholic acid in the treatment of primary biliary cirrhosis: results of a controlled study]. Zeitschrift fur Gastroenterologie. Verhandlungsband 1989;24:133. - PubMed
-
- Leuschner U, Fischer H, Kurtz W, Güldütuna S, Hubner K, Hellstern A, et al. Ursodeoxycholic acid in primary biliary cirrhosis: results of a controlled double‐blind trial. Gastroenterology 1989;97:1268‐74. - PubMed
Lindor 1994 {published and unpublished data}
-
- Angulo P, Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Dickson ER. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver 1999;19(2):115‐21. - PubMed
-
- Balan V, Dickson ER, Jorgensen RA, Lindor KD. Effect of ursodeoxycholic acid on serum lipids of patients with primary biliary cirrhosis [see comments]. Mayo Clinic Proceedings 1994;69:923‐9. - PubMed
-
- Batts KP, Jorgensen RA, Dickson ER, Hofmann AF, Rossi SS, Ludwig J, et al. The effects of ursodeoxycholic acid on hepatic inflammation and histological stage in patients with primary biliary cirrhosis (AASLD Abstract). Hepatology 1993;18:175A. - PubMed
-
- Batts KP, Jorgensen RA, Dickson ER, Lindor KD. Effects of ursodeoxycholic acid on hepatic inflammation and histological stage in patients with primary biliary cirrhosis. American Journal of Gastroenterology 1996;91:2314‐7. - PubMed
-
- Dickson ER, Lindor KD. Beneficial effects of ursodeoxycholic acid in an open trial of patients with primary biliary cirrhosis. Bile Acids as Therapeutic Agents. From Basic Science to Clinical Practice. Falk Symposium 58. 1991:271‐2.
Oka 1990 {published data only}
-
- Oka H, Toda G, Ikeda Y, Hashimoto N, Hasumura Y, Kamimura T, et al. A multicenter double‐blind controlled trial of ursodeoxycholic acid for primary biliary cirrhosis. Gastroenterologia Japonica 1990;25:774‐80. - PubMed
-
- Toda G, Oka H, Hasumura Y, Kamimura T, Ohat Y, Tsuji T, et al. A multicenter double‐blind controlled trial of ursodeoxycholic acid for primary biliary cirrhosis in Japan. XI International Bile Acid Meeting. Bile Acids as Therapeutic Agents ‐ From Basic Science to Clinical Practice. Freiburg. 1990:76.
Papatheodoridis 2002 {published data only}
-
- Hadziyannis S, Hadziyannis E. A randomised controlled trial of ursodeoxycholic acid (UDCA) in primary biliary cirrhosis (PBC) (AASLD Abstract). Hepatology 1988;8:1421.
-
- Hadziyannis SJ. Long‐term treatment of primary biliary cirrhosis with ursodeoxycholic acid: the third year of a controlled trial. XI International Bile Acid Meeting. Bile Acids as Therapeutic Agents ‐ From Basic Science to Clinical Practice. Freiburg. 1990:57‐8.
-
- Hadziyannis SJ, Hadziyannis ES, Lianidou E, Makris A. Long‐term treatment of primary biliary cirrhosis with ursodeoxycholic acid: the third year of a controlled trial. Bile Acids as Therapeutic Agents. From Basic Science to Clinical Practice. Falk Symposium 58. 1990:287‐96.
-
- Hadziyannis SJ, Hadziyannis ES, Makris A. A randomised controlled trial of ursodeoxycholic acid (UDCA) in primary biliary cirrhosis (PBC) [abstract]. Hepatology 1989;10:580.
-
- Hadziyannis SJ, Hadziyannis ES, Makris A. A randomised controlled trial of Ursodeoxyccholic acid (UDCA) in primary biliary cirrhosis (PBC). European Journal of Clinical Investigation 1989;19(Pt 2):A15.
Pares 2000 {published and unpublished data}
-
- Pares A, Caballeria L, Bruguera M, Rodes J. Factors influencing histological progression of early primary biliary cirrhosis. Effect of ursodeoxycholic acid. Journal of Hepatology 2001;34(Suppl 1):189‐90.
-
- Pares A, Caballeria L, Rodes J. Long‐term ursodeoxycholic acid treatment delays progression of mild primary biliary cirrhosis. Journal of Hepatology 2001;34(Suppl 1):187‐8.
-
- Parés A, Caballeria L, Rodes J, Bruguera M, Rodrigo L, Garcia‐Plaza A, et al. Long‐term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double‐blind controlled multicentric trial. Journal of Hepatology 2000;32:561‐6. - PubMed
-
- Parés A, for the Spanish Association for the Study of the Liver. Long‐term treatment of primary biliary cirrhosis with ursodeoxycholic acid: results of a randomised, double‐blind, placebo‐controlled trial (abstract). Journal of Hepatology 1997;26(Suppl 1):166.
Poupon 1991 {published data only}
-
- Calmus Y, Poupon R. Ursodeoxycholic acid (UDCA) in the treatment of chronic cholestatic diseases. Biochimie 1991;73:1335‐8. - PubMed
-
- Chazouilleres O, Legendre C, StMaur P, Ismail PR, Poupon R. Histological course of primary biliary cirrhosis (PBC) treated with ursodeoxycholic acid (UDCA) [abstract]. Hepatology 1995;2(4):125A.
-
- Corpechot C, Carrat F, Bonnand A‐M, Poupon RE, Poupon R. A Markov model for assessment of histological progression in PBC provides evidence that UDCA therapy is effective in early stages [abstract]. Hepatology 1999;30(4):474A.
-
- Corpechot C, Carrat F, Bonnand A‐M, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis. Hepatology 2000;32:1196‐9. - PubMed
-
- Degott C, Zafrani ES, Callard P, Balkau B, Poupon RE, Poupon R. Histopathologic study of primary biliary cirrhosis and the effect of ursodeoxycholic acid treatment on histological progression. Hepatology 1999;29:1007‐12. - PubMed
Senior 1991 {published data only}
-
- Arora R, Batta AK, Salen G, O'Brien C, Senior JR. Effect of ursodiol on bile acid conjugation in patients with primary biliary cirrhosis [AASLD abstract]. Hepatology 1990;12(4):994.
-
- Batta AK, Arora R, Salen G, O'Brian C, Senior JR. Effect of ursodiol on biliary bile acid composition and conjugation in patients with primary biliary cirrhosis. Gastroenterology 1990;98(2):A567.
-
- O'Brian CB, Senior JR, Sternlieb JM, Sample M, Saul SM, Arora R, et al. Ursodiol treatment of primary biliary cirrhosis. Gastroenterology 1990;98:A617.
-
- O'Brian CB, Senior JR, Sternlieb JM, Saul SM. Caution: not all patients with primary biliary cirrhosis may successfully be treated by ursodiol. Second International Meeting on Pathochemistry, Pathophysiology and Pathomechanisms of the Biliary System and New Strategies for the Treatment of Hepato‐Biliary Diseases. Bologna. 1990:208.
-
- Senior JR, O'Brian CB, Dickson ER. Effect of oral ursodiol treatment on the predicted probability of mortality in primary biliary cirrhosis. Hepatology 1990;12:438.
Turner 1994 {published and unpublished data}
-
- Myszor M, Turner I, Mitshison H, Bennett M, Burt AD, James OFW. No symptomatic or histological benefit from ursodeoxycholic acid treatment in PBC after 1 year. Controlled pilot study [IASL abstract]. Hepatology 1990;12:415.
-
- Turner IB, Myszor M, Mitchison HC, Bennett MK, Burt AD, James OF. A two year controlled trial examining the effectiveness of ursodeoxycholic acid in primary biliary cirrhosis. Journal of Gastroenterology and Hepatology 1994;9:162‐8. - PubMed
Vuoristo 1995 {published and unpublished data}
-
- Kisand KE, Karvonen A‐L, Vuoristo M, Färkkilä M, Lehtola J, Inkovaara J, et al. Ursodeoxycholic acid treatment lowers the serum levels of antibodies against pyruvate dehydrogenase and influences their inhibitory capacity for the enzyme complex in patients with primary biliary cirrhosis. Journal of Molecular Medicine 1996;74:269‐74. - PubMed
-
- Miettinen TA, Farkkila M, Vuoristo M, Karvonen AL, Leino R, Lehtola J, et al. Serum cholestanol, cholesterol precursors, and plant sterols during placebo‐controlled treatment of primary biliary cirrhosis with ursodeoxycholic acid or colchicine. Hepatology 1995;21:1261‐8. - PubMed
-
- Miettinen TA, Färkkila M, Vuoristo M, Karvonen A‐L, Leino R, Lehtola J, et al. Improvement of serum non cholesterol sterols may indicate retarded progression of primary biliary cirrhosis (PBC) in a randomised placebo controlled two‐year trial with colchicine and ursodeoxycholic acid (AASLD abstract). Gastroenterology 1993;104:A954.
-
- Vuoristo M, Farkkila M, Karvonen AL, Leino R, Lehtola J, Makinen J, et al. A placebo‐controlled trial of primary biliary cirrhosis treatment with colchicine and ursodeoxycholic acid [see comments]. Gastroenterology 1995;108:1470‐8. - PubMed
-
- Vuoristo M, Färkkilä M, Gylling H, Karvonen A‐L, Leino R, Lehtola J, et al. Expression and therapeutic response related to apolipoprotein E polymorphism in primary biliary cirrhosis. Journal of Hepatology 1997;27:136‐42. - PubMed
References to studies excluded from this review
Angulo 1999 {published data only}
-
- Angulo P, Batts KP, Therneau TM, Jorgensen RA, Dickson ER, Lindor KD. Long‐term ursodeoxycholic acid delays histological progression in primary biliary cirrhosis. Hepatology 1999;29(3):644‐7. - PubMed
Angulo 1999 a {published data only}
-
- Angulo P, Dickson ER, Therneau TM, Jorgensen RA, Smith C, DeSotel CK, et al. Comparison of three doses of ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a randomised trial. Journal of Hepatology 1999;30:830‐5. - PubMed
Bateson 1998 {published data only}
Brodanova 1997 {published data only}
-
- Brodanova M, Perlik F. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Casopis Lekaru Ceskych 1997;136(7):215‐20. - PubMed
Cauch‐Dudek 1998 {published data only}
Crippa 1995 {published data only}
-
- Crippa G, Cagnoni C, Castelli A, Concesi C, Girometta S, Pancotti D, et al. Prolonged treatment with ursodeoxycholic acid for primary biliary cirrhosis. Clinical Therapeutics 1995;146:367‐72. - PubMed
Crosignani 1996 {published data only}
-
- Crosignani A, Battezzati PM, Setchell KDR, Invernizzi P, Covini G, Zuin M, et al. Tauroursodeoxycholic acid for treatment of primary biliary cirrhosis. A dose‐response study. Digestive Diseases and Sciences 1996;41(4):809‐15. - PubMed
Eisenburg 1988 {published data only}
-
- Eisenburg J, Eder M, Spengler U, Berg PA, Caselmann W, Mannes AG, et al. Treatment of primary biliary cirrhosis with ursodeoxycholic acid. Part 2: Prospective long‐term trial in 21 patients. Fortschritte der Medizin 1988;106(34):695‐8. - PubMed
Ferri 1993 {published data only}
-
- Ferri F, Bernocchi P, Fedeli S. Tauroursodeoxycholic acid for treatment of primary biliary cirrhosis. A controlled comparison with ursodeoxycholic acid. Clinica Terapeutica 1993;143(4):321‐6. - PubMed
Grippa 1995 {published data only}
-
- Crippa G, Cagnoni C, Castelli A, Concesi C, Girometta S, Pancotti D, et al. Prolonged treatment with ursodeoxycholic acid for primary biliary cirrhosis. Clinica Terapeutica 1995;146(5):367‐72. - PubMed
Ideo 1990 {published data only}
-
- Idéo G, Bellati G, Pedraglio E, Bottelli R, Maggi G. Efficacy of ursodeoxycholic acid in lowering alanine aminotransferase and gamma‐glutamyl transpeptidase serum levels in patients with chronic active hepatitis and primary biliary cirrhosis. Current Therapeutic Research Clinical and Experimental 1990;47(1):62‐6.
Ikeda 1996 {published data only}
-
- Ikeda T, Tozuka S, Noguchi O, Kobayashi F, Sakamoto S, Marumo F, et al. Effects of additional administration of colchicine in ursodeoxycholic acid‐treated patients with primary biliary cirrhosis: A prospective randomised study. Journal of Hepatology 1996;24(1):88‐94. - PubMed
Kehagioglou 1991 {published data only}
-
- Kehagioglou K, Dritsas S, Kanatakis S, Tsatsa E, Mastora M, Chrissikos N, et al. Effect of UDCA on the natural course of PBC. Journal of Hepatology 1991;13(Suppl 2):S134.
Kim 1997 {published data only}
-
- Kim WR, Poterucha JJ, Jorgensen RA, Batts KP, Hombuger HA, Dickson‐ER, et al. Does antimitochondrial antibody status affect response to treatment in patients with primary biliary cirrhosis? Outcomes of ursodeoxycholic acid therapy and liver transplantation. Hepatology 1997;26(1):22‐6. - PubMed
Kneppelhout 1992 {published data only}
-
- Kneppelhout JC, Mulder CJJ, Berge Henegouwen GP, Vries RA, Brandt K‐H. Ursodeoxycholic acid treatment in primary biliary cirrhosis with the emphasis on late stage disease. Netherlands Journal of Medicine 1992;41(1):11‐6. - PubMed
Krzeski 1999 {published data only}
-
- Krzeski P, Habior A, Zych W, Walewska‐Zielecka B, Butruk E. Effects of ursodeoxycholic acid treatment on bilirubin concentration and survival of patients with primary biliary cirrhosis. Gastroenterologia Polska 1999;6(3):231‐4.
Larghi 1997 {published data only}
-
- Larghi A, Crosignani A, Battezzati PM, De‐Valle G, Allocca M, Invernizzi P, et al. Ursodeoxycholic and tauro‐ursodeoxycholic acids for the treatment of primary biliary cirrhosis: A pilot crossover study. Alimentary Pharmacology and Therapeutics 1997;11(2):409‐14. - PubMed
Leuschner 1996 {published data only}
-
- Leuschner M, Guldutuna S, You T, Hubner K, Bhatti S, Leuschner U. Ursodeoxycholic acid and prednisolone versus ursodeoxycholic acid and placebo in the treatment of early stages of primary biliary cirrhosis. Journal of Hepatology 1996;25(1):49‐57. - PubMed
LONDON 1998 {published data only}
-
- Verma A, Ahmed HA, Jazrawi RP, Davis T, Bland M, Benson M, et al. Determining the most efficacious dose of ursodeoxycholic acid in primary biliary cirrhosis (Abstract). XV International Bile Acid Meeting. Bile Acids and Cholestasis. Falk Symposium No 108. Titisee, Germany. 1998:62.
Lotterer 1990 {published data only}
-
- Lotterer E, Stiehl A, Raedsch R, Foelsch UR, Bircher J. Ursodeoxycholic acid in primary biliary cirrhosis: No evidence for toxicity in the stages I to III. Journal of Hepatology 1990;10(3):284‐90. - PubMed
Matsuzaka 1994 {published data only}
-
- Matsuzaki Y, Doy M, Tanaka N, Shoda J, Osuga T, Nakano M, et al. Biochemical and histological changes after more than four years of treatment of ursodeoxycholic acid in primary biliary cirrhosis. Journal of Clinical Gastroenterology 1994;18(1):36‐41. - PubMed
Matsuzaki 1990 {published data only}
-
- Matsuzaki Y, Tanaka N, Osuga T, Aikawa T, Shoda J, Doi M, et al. Improvement of biliary enzyme levels and itching as a result of long‐term administration of ursodeoxycholic acid in primary biliary cirrhosis. American Journal of Gastroenterology 1990;85(1):15‐23. - PubMed
MAYO‐II 1997 {published data only}
-
- Lindor KD, Jorgensen R, Theneau TM, Smith C, Mahoney DW, Dickson ER. Comparison of three different doses of ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a randomised trial. Hepatology 1997;26:438 A. - PubMed
NEWARK‐I {published data only}
-
- Batta AK, Arora R, Salen G, Katz S. Ursodeoxycholic acid improves liver function and reduces serum and urinary endogenous bile acids in primary biliary cirrhosis. Hepatology 1988;8:1221A.
-
- Batta AK, Arora R, Salen G, Tint GS, Eskreis D, Katz S. Characterization of serum and urinary bile acids in patients with primary biliary cirrhosis by gas‐liquid chromatography‐mass spectrometry: effect of ursodeoxycholic acid treatment. Journal of Lipid Research 1989;30:1953‐62. - PubMed
-
- Batta AK, Salen G, Arora R, Shefer S, Tint GS, Abroon J, et al. Effect of ursodeoxycholic acid on bile acid metabolism in primary biliary cirrhosis. Hepatology 1989;10:414‐9. - PubMed
-
- Eskreis D, Abroon J, Katz S, Salen G, Arora R. Ursodeoxycholic acid treatment of primary biliary cirrhosis. American Journal of Gastroenterology 1988;83:1065A.
NEWARK‐III {published data only}
-
- Batta AK, Salen G, Mirchandani R, Tint GS, Shefer S, Batta M, et al. Effect of long‐term treatment with ursodiol on clinical and biochemical features and biliary bile acid metabolism in patients with primary biliary cirrhosis. American Journal of Gastroenterology 1993;88:691‐700. - PubMed
Ogino 1993 {published data only}
-
- Ogino H, Unoura M, Kawai H, Terasaki S, Yanagi M, Matsushita E, et al. Effect of ursodeoxycholic acid therapy on lymphocyte function of patients with primary biliary cirrhosis. Acta Hepatologica Japonica 1993;34(4):306‐12.
Okuyama 1988 {published data only}
-
- Okuyama S, Higuchi T, Ichimiya H, Hayashi H, Sakamoto N. A case of primary biliary cirrhosis ‐ 4 years' treatment with 300 mg/day ursodeoxycholic acid. Acta Hepatologica Japonica 1988;29(6):799‐802.
Osuga 1989 {published data only}
-
- Osuga T, Tanaka N, Matsuzaki Y, Aikawa T. Effect of ursodeoxycholic acid in chronic hepatitis and primary biliary cirrhosis. Digestive Diseases and Sciences 1989;34(12 Suppl):49S‐51S. - PubMed
Peridigoto 1992 {published data only}
-
- Perdigoto R, Wiesner RH. Progression of primary biliary cirrhosis with ursodeoxycholic acid therapy. Gastroenterology 1992;102(4):1389‐91. - PubMed
Podda 1989 {published data only}
-
- Podda M, Ghezzi C, Battezzati PM, Bertolini E, Crosignan A, Petroni ML, et al. Ursodeoxycholic acid for chronic liver diseases. Journal of Clinical Gastroenterology 1988;10(Suppl 2):S25‐S31. - PubMed
-
- Podda M, Ghezzi C, Battezzati PM, Bertolini E, Crosignani A, Petroni ML, et al. Effect of different doses of ursodeoxycholic acid in chronic liver disease. Digestive Diseases and Sciences 1989;34(12 Suppl):59S‐65S. - PubMed
Poupon 1987 {published data only}
-
- Poupon R, Chrétien Y, Poupon RE, Ballet F, Calmus Y, Darnis F. Is ursodeoxycholic acid an effective treatment for primary biliary cirrhosis?. Lancet 1987;1(8537):834‐6. - PubMed
Poupon 1989 {published data only}
-
- Poupon R, Balkau B, Legendre C, Lévy VG, Chrétien Y, Poupon RE. Ursodeoxycholic acid improves histologic features and progression of primary biliary cirrhosis. Hepatology 1989;10(4):637.
Poupon 1996 {published data only}
-
- Poupon RE, Huet PM, Poupon R, Bonnand A‐M, Nhieu JT, Zafrani ES, et al. A randomised trial comparing colchicine and ursodeoxycholic acid combination to ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 1996;24(5):1098‐103. - PubMed
Schonfeld 1997 {published data only}
-
- Schonfeld JV, Breuer N, Zotz RB, Beste M, Goebell H. Serial quantitative liver function tests in patients with primary biliary cirrhosis: A prospective long‐term study. Digestion 1997;58(4):396‐401. - PubMed
Shibata 1992 {published data only}
-
- Shibata J, Fujiyama S, Honda Y, Sato T. Combination therapy with ursodeoxycholic acid and colchicine for primary biliary cirrhosis. Journal of Gastroenterology and Hepatology 1992;7(3):277‐82. - PubMed
Stiehl 1990 {published data only}
-
- Stiehl A, Rudolph G, Raedsch R, Moller B, Hopf U, Lotterer E, et al. Ursodeoxycholic acid‐induced changes of plasma and urinary bile acids in patients with primary biliary cirrhosis. Hepatology 1990;12(3 I):492‐7. - PubMed
Taha 1994 {published data only}
-
- Taha AS, Allison MC, Myara A, Trivin F, Duncan A, Russell RI. Does cholestyramine reduce the efficacy of ursodeoxycholic acid in primary biliary cirrhosis?. European Journal of Gastroenterology and Hepatology 1994;6(6):535‐8.
Takezaki 1991 {published data only}
-
- Takezaki E, Nishibayashi H, Murakami S, Kagawa K, Ohmori H, Kohda T, et al. A case of primary biliary cirrhosis with a histological improvement after long‐term therapy with ursodeoxycholic acid. IRYO Japanese Journal of National Medical Services 1991;45(4):376‐81.
Toda 1998 {published data only}
-
- Toda G, Tanaka N, Ikeda Y, Kobayashi K, Inoue K, Onji M, et al. Dose‐dependency of effect of ursodeoxycholic acid on primary biliary cirrhosis: a randomised, double‐blind controlled study. Kan‐Tan‐Sui (Japan) 1998;37:443‐60.
Unoura 1990 {published data only}
-
- Unoura M, Ogino H, Mizuno Y, Urabe T, Matsushita E, Kaneko S, et al. Effects of ursodeoxycholic acid on lymphocyte functions in primary biliary cirrhosis. XI International Bile Acid Meeting. Bile Acids as Therapeutic Agents ‐ From Basic Science to Clinical Practice. Freiburg. 1990:Abstract No. 77.
Van de Meeberg 1996 {published data only}
-
- Meeberg PC, Wolfhagen FH, Berge‐Henegouwen GP, Salemans JM, Tangerman A, Buuren HR, et al. Single or multiple dose ursodeoxycholic acid for cholestatic liver disease: biliary enrichment and biochemical response. Journal of Hepatology 1996;25:887‐94. - PubMed
Van Hoogstraten 1998 {published data only}
-
- Hoogstraten HJ, Smet MB, Renooij W, Breed JG, Engels LG, Ouden‐Muller JW, et al. A randomised trial in primary biliary cirrhosis comparing ursodeoxycholic acid in daily doses of either 10 mg/kg or 20 mg/kg. Dutch Multicentre PBC Study Group. Alimentary Pharmacology and Therapeutics 1998;12:965‐71. - PubMed
Verma 1999 {published data only}
-
- Verma A, Jazrawi RP, Ahmed HA, Davis T, Bland JM, Benson M, et al. Optimum dose of ursodeoxycholic acid in primary biliary cirrhosis. European Journal of Gastroenterology and Hepatology 1999;11(10):1069‐76. - PubMed
Wirth 1994 {published data only}
-
- Wirth HP, Meyenberger C, Altorfer J, Ammann R, Blum HE. Eosinophilia in primary biliary cirrhosis: Regression under therapy with ursodeoxycholic acid. Schweizerische Medizinische Wochenschrift 1994;124(19):810‐5. - PubMed
Wirth 1995 {published data only}
-
- Wirth HP, Zala G, Meyenberger Ch, Ammann R. Subtype pattern of antimitochondrial antibodies in primary biliary cirrhosis and response to ursodeoxycholic acid. Schweizerische Medizinische Wochenschrift 1995;125(15):750‐4. - PubMed
Wolfhagen 1994 {published data only}
-
- Wolfhagen FH, Buuren HR, Schalm SW. Combined treatment with ursodeoxycholic acid and prednisone in primary biliary cirrhosis. The Netherlands Journal of Medicine 1994;44:84‐90. - PubMed
Yamazaki 1992 {published data only}
-
- Yamazaki M, Morimoto H, Wakabayashi T, Suzuki K, Kida H, Sugioka G, et al. A patient with asymptomatic primary biliary cirrhosis associated with eosinophilic infiltration and peripheral eosinophilia improved by the administration of ursodeoxycholic acid. Acta Hepatologica Japonica 1992;33(4):348‐52.
Yamazaki 1996 {published data only}
-
- Yamazaki K, Nakadate I, Suzuki K, Sato S, Masuda T. Eosinophilia in primary biliary cirrhosis. American Journal of Gastroenterology 1996;91(3):516‐22. - PubMed
Yokomori 1996 {published data only}
-
- Yokomori H, Oda M, Kamegaya Y, Motoori T, Ohbu M, Ishii H. Rapid improvement of intractable pruritus in a case with primary biliary cirrhosis by a combined therapy of ursodeoxycholic acid (UDCA) and cholestyramine (CS) ‐ Serum bile acid analysis. Acta Hepatologica Japonica 1996;37(2):102‐8.
Additional references
AASLD 2010
-
- Silveira MG, Brunt EM, Heathcote J, Gores GJ, Lindor KD, Mayo MJ. American Association for the Study of Liver Diseases endpoints conference: design and endpoints for clinical trials in primary biliary cirrhosis. Hepatology 2010;52(1):349‐59. - PubMed
Ahrens 1950
-
- Ahrens EH Jr, Payne MA, Kunkel HG, Eisenmenger WJ, Blondheim SH. Primary biliary cirrhosis (classical article). Medicine‐Baltimore 1994;73(5):264‐80. - PubMed
Angulo 1999a
-
- Angulo P, Dickson ER, Therneau TM, Jorgensen RA, Smith C, DeSotel CK, Lange SM, Anderson ML, Mahoney DW, Lindor KD. Comparison of three doses of ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a randomised trial. Journal of Hepatology 1999;30(5):830‐5. - PubMed
Baker 2003
Begg 1994
-
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐1101. - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. - PubMed
Crosignani 1991
-
- Crosignani A, Podda M, Battezzati PM, Bertolini E, Zuin M, Watson D, Setchell KD. Changes in bile acid composition in patients with primary biliary cirrhosis induced by ursodeoxycholic acid administration. Hepatology 1991;14(6):1000‐7. - PubMed
DeMets 1987
-
- DeMets DL. Methods of combining randomised clinical trials: strengths and limitations. Statistics in Medicine 1987;6:341‐8. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Dickson 1989
-
- Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology 1989;10(1):1‐7. - PubMed
EASL 2009
-
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. Journal of Hepatology 2009;51(2):237‐67. - PubMed
Egger 1997
Egger 2003
-
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.. Health Technology Assessment 2003;7(1):1‐76. - PubMed
Gluud 2001a
-
- Gluud C, Christensen E. Ursodeoxycholic acid for primary biliary cirrhosis ‐ lessons for the future. Journal of Hepatology 2001;34(5):787‐8. - PubMed
Gluud 2006
-
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology 2006;163(6):493‐501. - PubMed
Gluud 2007
-
- Gluud C, Brok J, Gong Y, Koretz R. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46:734‐42. - PubMed
Gluud 2011
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2011, Issue 10. Art. No.: LIVER.
Goulis 1999
-
- Goulis J, Leandro G, Burroughs AK. Randomised controlled trials of ursodeoxycholic‐acid therapy for primary biliary cirrhosis: a meta‐analysis. Lancet 1999;354:1053‐60. - PubMed
Heathcote 2000
-
- Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology 2000;31(4):1005‐13. - PubMed
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. - PubMed
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmann1994
-
- Hofmann AF. Pharmacology of ursodeoxycholic acid, an enterohepatic drug. Scandinavian Journal of Gastroenterology. Supplement 1994;204:1‐15. - PubMed
Hollis 1999
ICH‐GCP 1997
-
- International Conference on Harmonisation of Technical Requiements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation & ICH Guidelines. Media: Parexel Barnett, 1997.
Jazrawi 1994
-
- Jazrawi RP, Caestecker JS, Goggin PM, Britten AJ, Joseph AEA, Maxwell JD, et al. Kinetics of hepatic bile acid handling in cholestatic liver disease: effect of ursodeoxycholic acid. Gastroenterogy 1994;106:134‐42. - PubMed
Kaplan 2005
-
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. New England Journal of Medicine 2005;353:1261‐73. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomised trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Kullak‐Ublick 2000
-
- Kullak‐Ublick GA, Stieger B, Hagenbuch B, Meier PJ. Hepatic transport of bile salts. Seminars in Liver Disease 2000;20:273‐92. - PubMed
Kürstein 2005
-
- Kürstein P, Gluud L, Willemann M, Olsen K, Kjellberg J, Sogaard J, et al. Agreement between reported use of interventions for liver diseases and research evidence in Cochrane systematic reviews. Journal of Hepatology 2005;43:984‐9. - PubMed
Lazaridis 2001
-
- Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid 'mechanisms of action and clinical use in hepatobiliary disorders. Journal of Hepatology 2001;35(1):134‐46. - PubMed
Lee 2005
-
- Lee YM, Kaplan MM. The natural history of PBC: has it changed?. Seminars in Liver Disease 2005;25(3):321‐6. - PubMed
Ludvig 1978
-
- Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Archiv. A. Pathological Anatomy and Histology 1978;379(2):103‐12. - PubMed
MacMahon 1949
-
- MacMahon HE, Thannhauser SJ. Xanthomatous biliary cirrhosis (a clinical syndrome). Annals of Internal Medicine 1949;30:121. - PubMed
Mayo 2005
-
- Mayo MJ. Patients and patience: the pitfalls of primary biliary cirrhosis trials. Nature Clinical Practise Gastroenterology & Hepatology 2005;2:552‐3. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. - PubMed
Myszor 1990
-
- Myszor M, Turner I, Mitshison H, Bennett M, Burt AD, James OFW. No symptomatic or histological benefit from ursodeoxycholic acid treatment in PBC after 1 year. Controlled pilot study [IASL abstract]. Hepatology 1990;12:415.
Pares 2006
-
- Pares A, Caballeria L, Rodes J. Excellent long‐term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology 2006;130(3):715‐20. - PubMed
Pasha 1999
-
- Pasha T, Heathcote J, Gabriel S, Cauch‐Dudek K, Jorgensen R, Therneau T, et al. Cost‐effectiveness of ursodeoxycholic acid therapy in primary biliary cirrhosis. Hepatology 1999;29:21‐6. - PubMed
Paumgartner 2002
-
- Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology 2002;36(3):525‐31. - PubMed
Poupon 1997
-
- Poupon RE, Lindor KD, Cauch‐Dudek K, Dickson RE, Poupon R, Heathcote JE. Combined analysis of randomised controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology 1997;113:884‐90. - PubMed
Poupon 2000
-
- Poupon RE. Ursodeoxycholic acid for primary biliary cirrhosis: lessons from the past ‐ issues for the future. Journal of Hepatology 2000;32:685‐8. - PubMed
Poupon 2010
-
- Poupon R. Primary biliary cirrhosis: a 2010 update. Journal of Hepatology 2010;52(5):745‐58.. - PubMed
Prince 2002
-
- Prince M, Chetwynd A, Newman W, Metcalf JV, James OFW. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow‐up for up to 28 years. Gatroenterology 2002;123:1044‐51. - PubMed
Prince 2003
-
- Prince MI, James OFW. The epidemiology of primary biliary cirrhosis. Clinics in Liver Disease 2003;7:795‐819. - PubMed
Prince 2004
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomised controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Rust 2005
-
- Rust C, Beuers U. Medical treatment of primary biliary cirrhosis and primary sclerosing cholangitis. Clinical Review in Allergy & Immunology 2005;28(2):135‐45. - PubMed
Saksena 1997
Savovic 2012
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, Als‐Nielsen B, Balk EM, Gluud C, Gluud LL, Ioannidis JP, Schulz KF, Beynon R, Welton NJ, Wood L, Moher D, Deeks JJ, Sterne JA. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Scheuer 1967
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes, R, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment in controlled trials. JAMA 1995;273:408‐12. - PubMed
Setchell 1996
Shapiro 1979
Sharp 1998
-
- Sharp SJ. Metaanalysis regression. STATA Techn Bull 1998:4216‐22.
Siegel 2003
-
- Siegel JL, Jorgensen R, Angulo P, Lindor KD. Treatment with ursodeoxycholic acid is associated with weight gain in patients with primary biliary cirrhosis. Journal of Clinical Gastroenterology 2003;37(2):183‐5. - PubMed
Simko 1994
-
- Simko V, Michael S, Prego V. Ursodeoxycholic therapy in chronic liver disease: a meta‐analysis in primary biliary cirrhosis and in chronic hepatitis. American Journal of Gastroenterology 1994;89:392‐8. - PubMed
Stiehl 1999
-
- Stiehl A, Benz C, Sauer P. Mechanism of hepatoprotective action of bile salts in liver disease. Gastroenterology Clinics of North America 1999;28(1):195‐209, viii. - PubMed
Talwalker 2003
-
- Talwalkar JA, Lindor KD. Primary biliary cirrhosis. Lancet 2003;362:53‐61. - PubMed
Thompson 2002
-
- Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21(11):1559‐73. - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
TSA manual 2011 [Computer program]
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA). Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research, 2011.
TSA program 2011 [Computer program]
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. Trial Sequential Analysis. Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research, 2011.
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
Wood 2008
References to other published versions of this review
Christensen 1997
-
- Christensen E, Gluud C. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 1997, Issue 4. - PubMed
Gluud 1999a
-
- Gluud C, Christensen E. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 1999, Issue 2. - PubMed
Gluud 1999b
-
- Gluud C, Christensen E. Ursodeoxycholic acid (UDCA) in primary biliary cirrhosis (PBC) ‐ a Cochrane Hepato‐Biliary systematic review. Journal of Hepatology 1999;30(Suppl 1):83.
Gluud 2001b
Gong 2008
-
- Gong Y, Huang ZB, Christensen E, Gluud C. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 2008;16(3):CD000551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

