Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients
- PMID: 23235609
- PMCID: PMC6464614
- DOI: 10.1002/14651858.CD004388.pub6
Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients
Abstract
Background: Sepsis is a common and frequently fatal condition. Human recombinant activated protein C (APC) has been introduced to reduce the high risk of death associated with severe sepsis or septic shock. This systematic review is an update of a Cochrane review originally published in 2007.
Objectives: We assessed the benefits and harms of APC for patients with severe sepsis or septic shock.
Search methods: We searched CENTRAL (The Cochrane Library 2012, Issue 6); MEDLINE (2010 to June 2012); EMBASE (2010 to June 2012); BIOSIS (1965 to June 2012); CINAHL (1982 to June 2012) and LILACS (1982 to June 2012). There was no language restriction.
Selection criteria: We included randomized clinical trials assessing the effects of APC for severe sepsis or septic shock in adults and children. We excluded studies on neonates. We considered all-cause mortality at day 28 and at the end of study follow up, and hospital mortality as the primary outcomes.
Data collection and analysis: We independently performed trial selection, risk of bias assessment, and data extraction in duplicate. We estimated relative risks (RR) for dichotomous outcomes. We measured statistical heterogeneity using the I(2) statistic. We used a random-effects model.
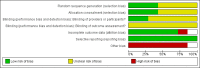
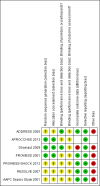
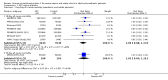
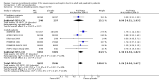
Main results: We identified one new randomized clinical trial in this update which includes six randomized clinical trials involving 6781 participants in total, five randomized clinical trials in adult (N = 6307) and one randomized clinical trial in paediatric (N = 474) participants. All trials had high risk of bias and were sponsored by the pharmaceutical industry. APC compared with placebo did not significantly affect all-cause mortality at day 28 compared with placebo (780/3435 (22.7%) versus 767/3346 (22.9%); RR 1.00, 95% confidence interval (CI) 0.86 to 1.16; I(2) = 56%). APC did not significantly affect in-hospital mortality (393/1767 (22.2%) versus 379/1710 (22.1%); RR 1.01, 95% CI 0.87 to 1.16; I(2) = 20%). APC was associated with an increased risk of serious bleeding (113/3424 (3.3%) versus 74/3343 (2.2%); RR 1.45, 95% CI 1.08 to 1.94; I(2) = 0%). APC did not significantly affect serious adverse events (463/3334 (13.9%) versus 439/3302 (13.2%); RR 1.04, 95% CI 0.92 to 1.18; I(2) = 0%). Trial sequential analyses showed that more trials do not seem to be needed for reliable conclusions regarding these outcomes.
Authors' conclusions: This updated review found no evidence suggesting that APC should be used for treating patients with severe sepsis or septic shock. APC seems to be associated with a higher risk of bleeding. The drug company behind APC, Eli Lilly, has announced the discontinuation of all ongoing clinical trials using this drug for treating patients with severe sepsis or septic shock. APC should not be used for sepsis or septic shock outside randomized clinical trials.
Conflict of interest statement
In 2004, Arturo Martí‐Carvajal was employed by Eli Lilly to run a four‐hour workshop on 'How to critically appraise clinical trials on osteoporosis and how to teach this'. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
In 2007, Arturo Martí‐Carvajal was employed by Merck to run a four‐hour workshop on 'How to critically appraise clinical trials and how to teach this'. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
Ivan Solà: none known.
Christian Gluud: none known.
Dimitrios Lathyris: none known.
Andrés Felipe: none known
Cardona: none known.
Figures


















Update of
-
Human recombinant activated protein C for severe sepsis.Cochrane Database Syst Rev. 2012 Mar 14;(3):CD004388. doi: 10.1002/14651858.CD004388.pub5. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2012 Dec 12;12:CD004388. doi: 10.1002/14651858.CD004388.pub6. PMID: 22419295 Updated.
References
References to studies included in this review
ADDRESS 2005 {published data only}
-
- Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. New England Journal of Medicine 2005;353(13):1332‐41. [MEDLINE: ] - PubMed
-
- Laterre PF, Abraham E, Janes JM, Trzaskoma BL, Correl NL, Booth F. ADDRESS (ADministration of DRotrecogin alfa[activated] in Early stage Severe sepsis) long‐term follow‐up: one year safety and efficacy evaluation. Critical Care Medicine 2007;35(6):1457‐63. [MEDLINE: ] - PubMed
APROCCHSS 2013 {published data only}
-
- Annane D, Timsit JF, Megarbane B, Martin C, Misset B, Mourvillier B, et al. Recombinant human activated protein C for adults with septic shock: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2013; Vol. Mar 22, issue Epub ahead of print. [PUBMED: 23525934] - PubMed
Dhainaut 2009 {published data only}
-
- Dhainaut JF, Antonelli M, Wright P, Desachy A, Reignier J, Lavoue S, et al. Extended drotrecogin alfa (activated) treatment in patients with prolonged septic shock. Intensive Care Medicine 2009;35(7):1187‐95. [MEDLINE: ] - PubMed
PROWESS 2001 {published data only}
-
- Angus DC, Linde‐Zwirble WT, Clermont G, Ball DE, Basson BR, Ely EW, et al. Cost‐effectiveness of drotrecogin alfa (activated) in the treatment of severe sepsis. Critical Care Medicine 2003;31(1):1‐11. [MEDLINE: ] - PubMed
-
- Barie PS, Williams MD, McCollam JS, Bates BM, Qualy RL, Lowry SF, et al. Benefit/risk profile of drotrecogin alfa (activated) in surgical patients with severe sepsis. American Journal of Surgery 2004;188(3):212‐20. [MEDLINE: ] - PubMed
-
- Bernard GR, Margolis BD, Shanies HM, Ely EW, Wheeler AP, Levy H, et al. Extended evaluation of recombinant human activated protein C United States Trial (ENHANCE US): a single‐arm, phase 3B, multicenter study of drotrecogin alfa (activated) in severe sepsis. Chest 2004;125(6):2206‐16. [MEDLINE: ] - PubMed
PROWESS‐SHOCK 2012 {published data only}
-
- Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, et al. Drotrecogin alfa (activated) in adults with septic shock. The New England Journal of Medicine 2012;366(22):2055‐64. [PUBMED: 22616830] - PubMed
-
- Ranieri VM, Thompson BT, Finfer S, Barie PS, Dhainaut JF, Douglas IS, et al. Unblinding plan of PROWESS‐SHOCK trial. Intensive Care Medicine 2011; Vol. 37, issue 8:1384‐5. [PUBMED: 21691888] - PubMed
RESOLVE 2007 {published data only}
-
- Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet 2007;369(9564):836‐43. [MEDLINE: ] - PubMed
rhAPC Sepsis Study 2001 {published data only}
-
- Bernard GR, Ely EW, Wright TJ, Fraiz J, Stasek JE Jr, Russell JA, et al. Safety and dose relationship of recombinant human activated protein C for coagulopathy in severe sepsis. Critical Care Medicine 2001;29(11):2051‐9. [MEDLINE: ] - PubMed
References to studies excluded from this review
Alaniz 2010 {published data only}
Barie 2011a {published data only}
-
- Barie PS, Hydo LJ, Shou J, Eachempati SR. Efficacy of therapy with recombinant human activated protein C of critically ill surgical patients with infection complicated by septic shock and multiple organ dysfunction syndrome. Surgical Infections. United States, 2011; Vol. 12, issue 6:443‐9. [PUBMED: 22185191] - PubMed
Barton 2004 {published data only}
-
- Barton P, Kalil AC, Nadel S, Goldstein B, Okhuysen Cawley R, et al. Safety, pharmacokinetics, and pharmacodynamics of drotrecogin alfa (activated) in children with severe sepsis. Pediatrics 2004;113(1 Pt 1):7‐17. [MEDLINE: ] - PubMed
Bearden 2002 {published data only}
-
- Bearden DT, Garvin CG. Recombinant human activated protein C for use in severe sepsis. Annals of Pharmacotherapy 2002;36(2):1424‐9. [ISSN: 1060‐0280] - PubMed
Bernard 2004 {published data only}
-
- Bernard GR, Margolis BD, Shanies HM, Ely EW, Wheeler AP, Levy H, et al. Extended evaluation of recombinant human activated protein C United States Trial (ENHANCE US): a single‐arm, phase 3B, multicenter study of drotrecogin alfa (activated) in severe sepsis. Chest 2004;125(6):2206‐16. [MEDLINE: ] - PubMed
Bertolini 2007 {published data only}
-
- Bertolini G, Rossi C, Anghileri A, Livigni S, Addis A, Poole D. Use of drotrecogin alfa (activated) in Italian intensive care units: The results of a nationwide survey. Intensive Care Medicine 2007;33(3):426‐34. [MEDLINE: ] - PubMed
Boyle 2012 {published data only}
-
- Boyle A, McKenzie C, Yassin S, McLuckie A, Wyncoll D. Adverse events and clinical outcome associated with drotrecogin alfa‐activated: A single‐center experience of 498 patients over 8 years. Journal of Critical Care 2012; Vol. 27, issue 3:320.e7‐320.e12. [PUBMED: 22172792] - PubMed
Casey 2002 {published data only}
-
- Casey M. Recombinant activated protein C (drotecogin alfa) in severe sepsis [Drotrecogin Alfa Activado (Proteína C activada recombinante) identificación de paciente e indicaciones para el tratamiento de la sepsis severa con alto riesgo de muerte]. Hematología (Buenos Aires) 2002;6:42‐6.
Costa 2007 {published data only}
Decruyenaere 2009 {published data only}
-
- Decruyenaere J, Backer D, Spapen H, Laterre PF, Raemaekers J, Rogiers P, et al. 90‐day follow‐up of patients treated with drotrecogin alfa (activated) for severe sepsis: a Belgian open label study. Acta Clinica Belgica 2009;64(1):16‐22. [MEDLINE: ] - PubMed
Ferrer 2009 {published data only}
-
- Ferrer R, Artigas A, Suarez D, Palencia E, Levy MM, Arenzana A, et al. Effectiveness of treatments for severe sepsis: A prospective multicenter observational study. American Journal of Respiratory and Critical Care Medicine 2009;180(9):861‐6. [MEDLINE: ] - PubMed
Freeman 2003 {published data only}
-
- Freeman BD, Zehnbauer BA, Buchman TG. A meta‐analysis of controlled trials of anticoagulant therapies in patients with sepsis. Shock 2003;20(1):5‐9. [MEDLINE: ] - PubMed
Goldstein 2006 {published data only}
-
- Goldstein B, Nadel S, Peters M, Barton R, Machado F, Levy H, et al. Enhance: Results of a global open‐label trial of drotrecogin alfa (activated) in children with severe sepsis. Pediatric Critical Care Medicine 2006;7(3):200‐11. [MEDLINE: ] - PubMed
Green 2005 {published data only}
-
- Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al. Clinical effectiveness and cost‐effectiveness of drotrecogin alfa (activated) (Xigris(R)) for the treatment of severe sepsis in adults: a systematic review and economic evaluation. Health Technology Assessment 2005;140:1‐126. [MEDLINE: ] - PubMed
Heslet 2004 {published data only}
-
- Heslet L, Nielsen JD, Schierbeck J, Andersen JS. Recombinant activated protein C: from evidence to clinical practice. Clinical practice guidelines for the use of activated proteins C in the treatment of severe sepsis. Ugeskrift for Laeger 2004;166(11):997‐1002. [MEDLINE: ] - PubMed
Houston 2009 {published data only}
-
- Houston G, Cuthbertson BH. Activated protein C for the treatment of severe sepsis. Clinical Microbiology and Infection 2009;5(4):319‐24. [MEDLINE: ] - PubMed
Kalil 2010a {published data only}
-
- Kalil A, Rosa S. Real‐life experience with drotrecogin alfa activated for severe sepsis: An effectiveness and safety meta‐analysis. Critical Care Medicine 2010;38:A117.
Kanji 2007 {published data only}
-
- Kanji S, Perreault MM, Chant C, Williamson D, Burry L. Evaluating the use of drotrecogin alfa (activated) in adult severe sepsis: a Canadian multicenter observational study. Intensive Care Medicine 2007;33(3):517‐33. [MEDLINE: ] - PubMed
Khan 2010 {published data only}
-
- Khan A, Agarwal R, Aggarwal AN, Gupta D. Prevalence of serious bleeding events and intracranial hemorrhage in patients receiving activated protein C: A systematic review and meta‐analysis. Respiratory Care 2010;55(7):901‐10. [PUBMED: 20587103] - PubMed
Kylat 2012 {published data only}
Levi 2007 {published data only}
-
- Levi M, Levy M, Williams MD, Douglas I, Artigas A, Antonelli M, et al. Prophylactic HepaRin Evaluation in Severe Sepsis (XPRESS) Study Group. Prophylactic heparin in patients with severe sepsis treated with drotrecogin alfa (activated). American Journal of Respiratory and Critical Care Medicine 2007;176(5):483‐90. [MEDLINE: ] - PubMed
Lopez 2010 {published data only}
-
- Lopez Sanchez F, Mas‐Serrano P, Caturla Such J, Mignini M, Navarro J. Drotrecogin alfa (activated) in real‐life clinical practice for management of severe sepsis in surgical patients. Intensive Care Medicine 2010;36:S193.
Lucioni 2002 {published data only}
-
- Lucioni C, Mazzi S, Christopher C. The treatment of patients with severe sepsis using drotrecogin alfa: an economic evaluation for Italy [Il trattamento di pazienti con sepsi grave mediante drotrecogin alfa: una valutazione economica con riferimento allItalia]. Farmeconomia e Percorsi Terapeutici 2002;3(3):171‐7.
Marraro 2009 {published data only}
-
- Marraro GA. Treatment of septic shock and use of drotrecogin alfa (activated) in children. Expert Review of Anti‐Infective Therapy 2009;7(7):769‐72. [EMBASE: 2009575232] - PubMed
McCoy 2003 {published data only}
-
- McCoy C, Matthews SJ. Drotrecogin alfa (recombinant human activated protein C) for the treatment of severe sepsis. Clinical Therapeutics 2003;25(2):396‐421. [MEDLINE: ] - PubMed
McCoy 2004 {published data only}
-
- McCoy C. Safety of drotrecogin alfa (activated) in the treatment of patients with severe sepsis. Expert Opinion on Drug Safety 2004;3(6):625‐37. [MEDLINE: ] - PubMed
Neilson 2003 {published data only}
-
- Neilson AR, Burchardi H, Chinn C, Clouth J, Schneider H, Angus D. Cost‐effectiveness of drotrecogin alfa (activated) for the treatment of severe sepsis in Germany. Journal of Critical Care 2003;18(4):217‐27. [MEDLINE: ] - PubMed
Sadique 2011 {published data only}
Sanchez 2010 {published data only}
-
- Sanchez B, Piacentini E, Pradella V, Mignini M, Nava J. Hemodynamic effects of recombinant human activated protein C in patients with septic shock. Journal of Critical Care 2010;25(2):343‐7. [PUBMED: 19781904] - PubMed
Sanchez‐Garcia 2011 {published data only}
-
- Sanchez‐Garcia E, Navarro‐Martinez JA, Idrissi A, Paya E, Nicolas N, Mas‐Serrano P. Use of drotrecogin alpha in a surgical cohort of intra‐abdominal infections patients. European Journal of Anaesthesiology 2011;28:182.
Shorr 2010 {published data only}
-
- Shorr AF, Janes JM, Artigas A, Tenhunen J, Wyncoll DL, Mercier E, et al. Randomized trial evaluating serial protein C levels in severe sepsis patients treated with variable doses of drotrecogin alfa (activated). Critical Care 2010; Vol. 14, issue 6:R229. [CENTRAL: CN‐00802129; PUBMED: 21176144] - PMC - PubMed
van Doorn 2008 {published data only}
-
- Doorn KJ, Spapen H, Geers C, Diltoer M, Shabana W. Sepsis‐related acute kidney injury: a protective effect of drotrecogin alpha (activated) treatment?. Acta Anaesthesiologica Scandinavica 2008;52(9):1259‐64. [MEDLINE: ] - PubMed
Vincent 2005 {published data only}
-
- Vincent JL, Bernard GR, Beale R, Doig C, Putensen C, Dhainaut JF, et al. Drotrecogin alfa (activated) treatment in severe sepsis from the global open‐label trial ENHANCE: Further evidence for survival and safety and implications for early treatment. Critical Care Medicine 2005;33(10):2266‐77. [MEDLINE: ] - PubMed
Wheeler 2008 {published data only}
-
- Wheeler A, Steingrub J, Schmidt GA, Sanchez P, Jacobi J, Linde Zwirble W, et al. A retrospective observational study of drotrecogin alfa (activated) in adults with severe sepsis: comparison with a controlled clinical trial. Critical Care Medicine 2008;36(1):14‐23. [MEDLINE: ] - PubMed
Additional references
ACCP‐SCCM 1992
-
- No authors listed. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Critical Care Medicine 1992;20(6):864‐74. [MEDLINE: ] - PubMed
Angus 2001a
-
- Angus DC, Wax RS. Epidemiology of sepsis: An update. Critical Care Medicine 2001;29 Suppl 7:109‐16. [MEDLINE: ] - PubMed
Angus 2001b
-
- Angus DC, Linde‐Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated cost of care. Critical Care Medicine 2001;29(7):1303‐10. [MEDLINE: ] - PubMed
Annane 2005
-
- Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet 2005;365(9453):63‐78. [MEDLINE: ] - PubMed
Azevedo 2008
-
- Azevedo LC, Park M, Schettino GP. Novel potential therapies for septic shock. Shock 2008;30 Suppl 1:60‐6. [MEDLINE: ] - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] - PubMed
Barnato 2008
Bekelman 2003
-
- Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA 2003;289(4):454‐65. [MEDLINE: ] - PubMed
Bernard 2001
-
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez‐Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. New England Journal Medicine 2001;344(10):699‐709. [MEDLINE: ] - PubMed
Bhandari 2004
Bochud 2001
-
- Bochud PY, Glauser MP, Calandra T, International Sepsis Forum. Antibiotics in sepsis. Intensive Care Medicine 2001;27 Suppl 1:33‐48. [MEDLINE: ] - PubMed
Bochud 2004
-
- Bochud PY, Bonten M, Marchetti O, Calandra T. Antimicrobial therapy for patients with severe sepsis and septic shock: An evidence‐based review. Critical Care Medicine 2004;32(11 Suppl):495‐512. [MEDLINE: ] - PubMed
Bone 1992
-
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101(6):1644‐55. [MEDLINE: ] - PubMed
Boussekey 2008
-
- Boussekey N, Chiche A, Faure K, Devos P, Guery B, D'Escrivan T, et al. A pilot randomized study comparing high and low volume hemofiltration on vasopressor use in septic shock. Intensive Care Medicine 2008;34(9):1646‐53. [MEDLINE: ] - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [PUBMED: 18411040] - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive‐‐Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] - PubMed
Brun‐Buisson 1995
-
- Brun‐Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, et al. Incidence, risk factors and outcomes of severe sepsis and septic shock in adults: A multicenter prospective study in intensive care units. JAMA 1995;274(12):968‐74. [MEDLINE: ] - PubMed
Brun‐Buisson 2006
-
- Brun‐Buisson C. Epidemiology of severe sepsis [Épidémiologie des états septiques graves]. Presse Médicale 2006;35(3 Pt 2):513‐20. [MEDLINE: ] - PubMed
Carlet 2008
-
- Carlet J, Cohen J, Calandra T, Opal SM, Masur H. Sepsis: time to reconsider the concept. Critical Care Medicine 2008;36(3):964‐6. [MEDLINE: ] - PubMed
Cheng 2007
-
- Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M, et al. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Critical Care Medicine 2007;35(11):2538‐46. [MEDLINE: ] - PubMed
Christiaans 2013
-
- Christiaans SC, Wagener BM, Esmon CT, Pittet JF. Protein C and acute inflammation: a clinical and biological perspective. American Journal of Physiology. Lung Cellular and Molecular Physiology 2013;305(7):L455‐66. [PUBMED: 23911436] - PubMed
Cinel 2009
-
- Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Critical Care Medicine 2009;37(1):291‐304. [MEDLINE: ] - PubMed
Cohen 2009
-
- Cohen J. Non‐antibiotic strategies for sepsis. Clinical Microbiology and Infection 2009;15(4):302‐7. [MEDLINE: ] - PubMed
Cohen 2004
-
- Cohen J, Brun‐Buisson C, Torres A, Jorgensen J. Diagnosis of infection in sepsis: An evidence‐based review. Critical Care Medicine 2004;32(11):466‐94. [MEDLINE: ] - PubMed
CTU 2011 [Computer program]
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. Trial Sequential Analysis. Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research, 2011.
Cunneen 2004
-
- Cunneen J, Cartwright M. The puzzle of sepsis: fitting the pieces of the inflammatory response with treatment. AACN Clinical Issues 2004;15(1):18‐44. [MEDLINE: ] - PubMed
Cunnington 2008
-
- Cunnington A, Nadel S. New therapies for sepsis. Current Topics in Medicinal Chemistry 2008;8(7):603‐14. [MEDLINE: ] - PubMed
Danai 2007
-
- Danai PA, Sinha S, Moss M, Haber MJ, Martin GS. Seasonal variation in the epidemiology of sepsis. Critical Care Medicine 2007;35(2):410‐5. [MEDLINE: ] - PubMed
Das 2000
Deeks 2003
-
- Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non‐randomised intervention studies. Health Technology Assessment 2003;7(27):iii‐x, 1‐173. [PUBMED: 14499048] - PubMed
Dellinger 2008
EMEA 2005
-
- European Medicines Agency´s Committee for Medicinal Products for Human Use. Drotrecogin alfa (activated) only for use in high risk patients. EMEA's post‐authorisation summary of opinion for Xigris, EMEA/138447/2005, 21 April 2005. www.emea.europa.eu/ (accessed 29 November 2010).
EMEA 2011
-
- European Medicines Agency. Xigris (drotrecogin alfa (activated)) to be withdrawn due to lack of efficacy. Press Release 25 October 2011; Vol. EMA/856472/2011 Press Office:1‐2.
Esmon 2006
-
- Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Seminars in Thrombosis and Hemostasis 2006;32 Suppl 1:49‐60. [MEDLINE: ] - PubMed
FDA 2001a
-
- Food, Drug Administration. FDA Anti‐Infective Drugs Advisory Committee12 September 2001: Briefing Document for XIGRIS™ for the Treatment of Severe Sepsis. Eli Lilly and Company. 06 August 2001. Available for public disclosure without redaction.. http://www.fda.gov/ohrms/dockets/AC/01/briefing/3797b1_01_Sponsor.pdf (accessed 10 June 2009).
FDA 2001b
-
- Food, Drug Administration. FDA Briefing document: Anti‐infective Advisory Committee. Drotrecogin alfa (activated)). [Recombinant human activated protein (rhAPC)]. XIGRIS™ BLA # 125029/0.. http://www.fda.gov/ohrms/dockets/ac/01/briefing/3797b1_02_FDAbriefing.pdf (accessed 21 September 2005).
FDA 2005
-
- Food, Drug Administration. MedWatch ‐ Xigris [drotrecogin alfa (activated)]. www.fda.gov/medwatch/safety/2005/xigris_dearHCP_4‐21‐05.htm [Posted 01‐07‐2005] (accessed 21 September 2005).
Finfer 2008
Fisher 2000
-
- Fisher CJ, Yan SB. Protein C levels as a prognostic indicator of outcome in sepsis and related diseases. Critical Care Medicine 2000;28 Suppl 9:49‐56. [MEDLINE: ] - PubMed
Garnacho 2003
-
- Garnacho‐Montero J, Garcia‐Garmendia JL, Barrero‐Almodovar A, Jimenez‐Jimenez FJ, Perez‐Paredes C, Ortiz‐Leyba C. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Critical Care Medicine 2003;31(12):2742‐51. [MEDLINE: ] - PubMed
Garnacho 2006
Geroulanos 2006
-
- Geroulanos S, Douka ET. Historical perspective of the word "sepsis". Intensive Care Medicine 2006;32(12):2077. [MEDLINE: ] - PubMed
Girbes 2008
-
- Girbes AR, Beishuizen A, Strack van Schijndel RJ. Pharmacological treatment of sepsis. Fundamental and Clinical Pharmacology 2008;22(4):355‐61. [MEDLINE: ] - PubMed
Goldstein 2005
-
- Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatric Critical Care Medicine 2005;6(1):2‐8. [MEDLINE: ] - PubMed
Guidet 2005
-
- Guidet B, Aegerter P, Gauzit R, Meshaka P, Dreyfuss D, CUB‐Réa Study Group. Incidence and impact of organ dysfunctions associated with sepsis. Chest 2005;127(3):942‐51. [MEDLINE: ] - PubMed
Guyatt 2011
-
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] - PubMed
Guyatt 2011a
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐‐study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence‐‐indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] - PubMed
Guyatt 2011e
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] - PubMed
Guyatt 2011f
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] - PubMed
Guyatt 2011g
-
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence‐‐publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] - PubMed
Healy 2002
-
- Healy DP. New and emerging therapies for sepsis. Annals of Pharmacotherapy 2002;36(4):648‐54. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2009. Available from www.cochrane‐handbook.org.
Hinds 2001
Holmes 2003
-
- Holmes CL, Russell JA, Walley KR. Genetic polymorphisms in sepsis and septic shock: role in prognosis and potential for therapy. Chest 2003;124(3):1103‐15. [MEDLINE: ] - PubMed
Hotchkiss 2010
Iannaro 2009
-
- Ianaro A, Tersigni M, D'Acquisto F. New insight in LPS antagonist. Mini Reviews in Medicinal Chemistry 2009;9(3):306‐17. [MEDLINE: ] - PubMed
ICH‐GCP1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Ioannidis 2010
-
- Ioannidis JP. The art of getting it wrong. Research Synthesis Methods 2010;1(3‐4):169‐84. - PubMed
Iñigo 2006
-
- Iñigo J, Sendra JM, Díaz R, Bouza C, Sarría‐Santamera A. Epidemiology and costs of severe sepsis in Madrid. A hospital discharge study [Epidemiología y costes de la sepsis grave en Madrid.Estudio de altas hospitalarias]. Medicina Intensiva 2006;30(5):197‐203. [MEDLINE: ] - PubMed
Jørgensen 2006
Kalil 2012
-
- Kalil AC, LaRosa SP. Effectiveness and safety of drotrecogin alfa (activated) for severe sepsis: a meta‐analysis and metaregression. The Lancet Infectious Diseases 2012;12(9):678‐86. [PUBMED: 22809883] - PubMed
Khan 2008
-
- Khan SN, Mermer MJ, Myers E, Sandhu HS. The roles of funding source, clinical trial outcome, and quality of reporting in orthopedic surgery literature. The American Journal of Orthopedic Surgery 2008;37(12):205‐12. [MEDLINE: ] - PubMed
Khwannimit 2009
-
- Khwannimit B, Bhurayanontachai R. The epidemiology of, and risk factors for, mortality from severe sepsis and septic shock in a tertiary‐care university hospital setting. Epidemiology and Infection 2009;137(9):1‐9. [MEDLINE: ] - PubMed
King 2013
Kumar 2004
-
- Kumar A, Mann HJ. Appraisal of four novel approaches to the prevention and treatment of sepsis. American Journal of Health‐System Pharmacists 2004;61(8):765‐74. [MEDLINE: ] - PubMed
Leon 2008
Leone 2010
-
- Leone M, Textoris J, Michel F, Wiramus S, Martin C. Emerging drugs in sepsis. Expert Opinion on Emerging Drugs 2010;15(1):41‐52. [EMBASE: 2010160115; MEDLINE: ] - PubMed
Levi 2008
-
- Levi M. The coagulant response in sepsis. Clinics in Chest Medicine 2008;29(45):627‐42. [MEDLINE: ] - PubMed
Lexchin 2003
Lexchin 2005
-
- Lexchin JR. Implications of pharmaceutical industry funding on clinical research. Annals of Pharmacotherapy 2005;39(1):194‐7. [MEDLINE: ] - PubMed
Mann 2002
-
- Mann HJ. Recombinant human activated protein C in severe sepsis. American Journal of Health‐System Pharmacy 2002;59 Suppl 1:19‐23. [MEDLINE: ] - PubMed
Mann 2009
-
- Mann HJ, Short MA, Schlichting DE. Protein C in critical illness. American Journal of Health‐System Pharmacy 2009;66(12):1089‐96. [MEDLINE: ] - PubMed
Marshall 2004
-
- Marshall JC, Maier RV, Jimenez M, Dellinger EP. Source control in the management of severe sepsis and septic shock: an evidence‐based review. Critical Care Medicine 2004;32 Suppl 11:513‐26. [MEDLINE: ] - PubMed
Marshall 2008
-
- Marshall JC. Sepsis: Rethinking the approach to clinical research. Journal of Leukocyte Biology 2008;83(3):471‐82. [MEDLINE: ] - PubMed
Matsuda 2007
-
- Matsuda N, Hattori Y. Vascular biology in sepsis: pathophysiological and therapeutic significance of vascular dysfunction. Journal of Smooth Muscle Research 2007;43(4):117‐37. [MEDLINE: ] - PubMed
Melander 2003
Mitka 2011
-
- Mitka M. Drug for severe sepsis is withdrawn from market, fails to reduce mortality. JAMA 2011; Vol. 306, issue 22:2439‐40. [PUBMED: 22166598] - PubMed
Moine 2007
-
- Moine P. Potential benefits of non‐anti‐infective treatments of septic shock: a critical analysis of literature. Annales Francaises D'Anesthesie Et De Reanimation 2007;26(4):370‐5. [MEDLINE: ] - PubMed
Parrish 2008
-
- Parrish WR, Gallowitsch‐Puerta M, Czura CJ, Tracey KJ. Experimental therapeutic strategies for severe sepsis: mediators and mechanisms. Annals of the New York Academy of Sciences 2008;1144:210‐36. [MEDLINE: ] - PubMed
Paul 2009
-
- Paul M. Is there a treatment for sepsis other than antibiotics?. Clinical Microbiology and Infection 2009;15(4):297. [MEDLINE: ] - PubMed
Perner 2012
-
- Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, et al. Hydroxyethyl starch 130/0.4 versus Ringer's acetate in severe sepsis. The New England journal of medicine 2012;June 27:[Epub ahead of print]. [PUBMED: 22738085] - PubMed
Porta 2008
-
- Porta M. A Dictionary of Epidemiology. Fifth. New York: Oxford University Press, 2008.
Rangel‐Frausto 1995
-
- Rangel‐Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 1995;273(2):117‐23. [MEDLINE: ] - PubMed
Remick 2007
RevMan 5.1 [Computer program]
-
- The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2011.
Rhodes 2004
-
- Rhodes A, Bennett ED. Early goal‐directed therapy: An evidence‐based review. Critical Care Medicine 2004;32 Suppl 11:448‐50. [MEDLINE: ] - PubMed
Rigato 2006
-
- Rigato O, Silva E, Salomao R. Pathogenesis‐oriented targets for adjunctive therapy. Endocrine Metabolic & Immune Disorders‐Drug Targets 2006;6(2):193‐200. [MEDLINE: ] - PubMed
Rittirsch 2008
Sandrock 2010
-
- Sandrock CE, Albertson TE. Controversies in the treatment of sepsis. Seminars in Respiratory and Critical Care Medicine 2010;31(1):66‐78. [EMBASE: 2010112081; MEDLINE: ] - PubMed
Schuerholz 2008
-
- Schuerholz T, Marx G. Management of sepsis. Minerva Anestesiologica 2008;74(5):181‐95. [MEDLINE: ] - PubMed
Shorr 2006
Shorr 2008
Sibila 2008
-
- Sibila O, Agustí C, Torres A. Corticosteroids in severe pneumonia. The European Respiratory Journal 2008;32(2):259‐64. [MEDLINE: ] - PubMed
Sipahi 2006
-
- Sipahi T, Pocan H, Akar N. Effect of various genetic polymorphisms on the incidence and outcome of severe sepsis. Clinical and Applied Thrombosis and Hemostasis 2006;12(1):47‐54. [MEDLINE: ] - PubMed
Sismondo 2008a
-
- Sismondo S. How pharmaceutical industry funding affects trial outcomes: causal structures and responses. Social Science & Medicine 2008;66(9):1909‐14. [MEDLINE: ] - PubMed
Sismondo 2008b
-
- Sismondo S. Pharmaceutical company funding and its consequences: a qualitative systematic review. Contemporary Clinical Trials 2008;29(2):109‐13. [MEDLINE: ] - PubMed
Sriskandan 2008
-
- Sriskandan S, Altmann DM. The immunology of sepsis. The Journal of Pathology 2008;214(2):211‐23. [MEDLINE: ] - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [PUBMED: 18824467] - PubMed
Thorlund 2010
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). http://ctu.dk/tsa/files/tsa_manual.pdf (accessed 30 April 2012). 2011.
Treacher 2009
-
- Treacher DF, Alun Brown K. The basic science of sepsis. Surgery 2009;27(11):465‐9. [EMBASE: 2009641439]
Vincent 2002
Vincent 2004
-
- Vincent JL. Evidence‐based medicine in the ICU: important advances and limitations. Chest 2004;126(2):592‐600. [MEDLINE: ] - PubMed
Vincent 2013
-
- Vincent JL, Backer D. Circulatory shock. New England Journal of Medicine 2013;369(18):1726‐34. [PUBMED: 24171518] - PubMed
Wang 2009a
Wang 2009b
Wesche‐Soldato 2007
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] - PubMed
Wetterslev 2009
Wittebole 2008
-
- Wittebole X, Collienne C, Castanares‐Zapatero D, Laterre PF. Adjunctive therapies for severe sepsis. International Journal of Antimicrobial Agents 2008;32 Suppl 1:34‐8. [MEDLINE: ] - PubMed
Yan 2001
-
- Yan SB, Dhainaut JF. Activated protein C versus protein C in severe sepsis. Critical Care Medicine 2001;29(7 Suppl):69‐74. [MEDLINE: ] - PubMed
Zavala 2006 [Computer program]
-
- Zavala D, Martí‐Carvajal A, Peña‐Martí G, Comunián G. Data extraction sheet to help manage the characteristics of included studies in Cochrane reviews. Valencia: Universidad de Carabobo, 2006.
References to other published versions of this review
Marti‐Carvajal 2012
Martí‐Carvajal 2007
Martí‐Carvajal 2008
Martí‐Carvajal 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

