Interventions for actinic keratoses
- PMID: 23235610
- PMCID: PMC6599879
- DOI: 10.1002/14651858.CD004415.pub2
Interventions for actinic keratoses
Abstract
Background: Actinic keratoses are a skin disease caused by long-term sun exposure, and their lesions have the potential to develop into squamous cell carcinoma. Treatments for actinic keratoses are sought for cosmetic reasons, for the relief of associated symptoms, or for the prevention of skin cancer development. Detectable lesions are often associated with alteration of the surrounding skin (field) where subclinical lesions might be present. The interventions available for the treatment of actinic keratoses include individual lesion-based (e.g. cryotherapy) or field-directed (e.g. topical) treatments. These might vary in terms of efficacy, safety, and cosmetic outcomes.
Objectives: To assess the effects of topical, oral, mechanical, and chemical interventions for actinic keratosis.
Search methods: We searched the following databases up to March 2011: the Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library, MEDLINE (from 2005), EMBASE (from 2010), and LILACS (from 1982). We also searched trials registers, conference proceedings, and grey literature sources.
Selection criteria: Randomised controlled trials (RCTs) comparing the treatment of actinic keratoses with either placebo, vehicle, or another active therapy.
Data collection and analysis: At least two authors independently abstracted data, which included adverse events, and assessed the quality of evidence. We performed meta-analysis to calculate a weighted treatment effect across trials, and we expressed the results as risk ratios (RR) and 95% confidence intervals (CI) for dichotomous outcomes (e.g. participant complete clearance rates), and mean difference (MD) and 95% CI for continuous outcomes (e.g. mean reduction in lesion counts).
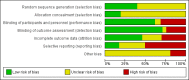
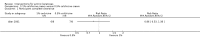
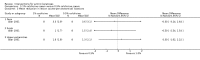
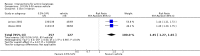
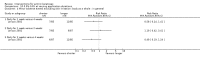
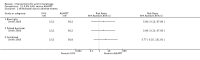
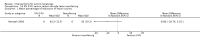
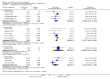
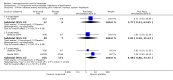
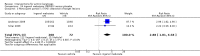
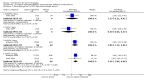
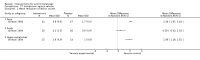
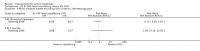
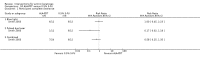
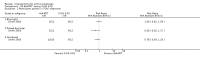
Main results: We included 83 RCTs in this review, with a total of 10,036 participants. The RCTs covered 18 topical treatments, 1 oral treatment, 2 mechanical interventions, and 3 chemical interventions, including photodynamic therapy (PDT). Most of the studies lacked descriptions of some methodological details, such as the generation of the randomisation sequence or allocation concealment, and half of the studies had a high risk of reporting bias. Study comparison was difficult because of the multiple parameters used to report efficacy and safety outcomes, as well as statistical limitations. We found no data on the possible reduction of squamous cell carcinoma.The primary outcome 'participant complete clearance' significantly favoured four field-directed treatments compared to vehicle or placebo: 3% diclofenac in 2.5% hyaluronic acid (RR 2.46, 95% CI 1.66 to 3.66; 3 studies with 420 participants), 0.5% 5-fluorouracil (RR 8.86, 95% CI: 3.67 to 21.44; 3 studies with 522 participants), 5% imiquimod (RR 7.70, 95% CI 4.63 to 12.79; 9 studies with1871 participants), and 0.025% to 0.05% ingenol mebutate (RR 4.50, 95% CI 2.61 to 7.74; 2 studies with 456 participants).It also significantly favoured the treatment of individual lesions with photodynamic therapy (PDT) compared to placebo-PDT with the following photosensitisers: aminolevulinic acid (ALA) (blue light: RR 6.22, 95% CI 2.88 to 13.43; 1 study with 243 participants, aminolevulinic acid (ALA) (red light: RR 5.94, 95% CI 3.35 to 10.54; 3 studies with 422 participants), and methyl aminolevulinate (MAL) (red light: RR 4.46, 95% CI 3.17 to 6.28; 5 studies with 482 participants). ALA-PDT was also significantly favoured compared to cryotherapy (RR 1.31, 95% CI 1.05 to 1.64).The corresponding comparative risks in terms of number of participants completely cleared per 1000 were as follows: 313 with 3% diclofenac compared to 127 with 2.5% hyaluronic acid; 136 with 0.5% 5-fluorouracil compared to 15 with placebo; 371 with 5% imiquimod compared to 48 with placebo; 331 with ingenol mebutate compared to 73 with vehicle; 527 to 656 with ALA/MAL-PDT treatment compared to 89 to 147 for placebo-PDT; and 580 with ALA-PDT compared to 443 with cryotherapy.5% 5-fluorouracil efficacy was not compared to placebo, but it was comparable to 5% imiquimod (RR 1.85, 95% Cl 0.41 to 8.33).A significant number of participants withdrew because of adverse events with 144 participants affected out of 1000 taking 3% diclofenac in 2.5% hyaluronic acid, compared to 40 participants affected out of 1000 taking 2.5% hyaluronic acid alone, and 56 participants affected out of 1000 taking 5% imiquimod compared to 21 participants affected out of 1000 taking placebo.Based on investigator and participant evaluation, imiquimod treatment and photodynamic therapy resulted in better cosmetic outcomes than cryotherapy and 5-fluorouracil.
Authors' conclusions: For individual lesions, photodynamic therapy appears more effective and has a better cosmetic outcome than cryotherapy. For field-directed treatments, diclofenac, 5-fluorouracil, imiquimod, and ingenol mebutate had similar efficacy, but their associated adverse events and cosmetic outcomes are different. More direct comparisons between these treatments are needed to determine the best therapeutic approach.
Conflict of interest statement
Dr Aditya Gupta, lead author of this review, participated in a clinical trial sponsored by DUSA in 2004, which was excluded from the review because it did not meet the inclusion criteria.
Dr Stephen Keohane, clinical referee for this review, states: "I have been paid for lectures and advisory boards by Galderma, Almirall, Intendis, Inc., Shire, and Bayer."
Figures




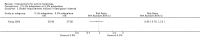




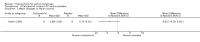
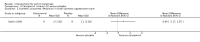
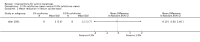

















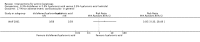

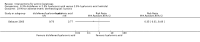



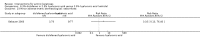

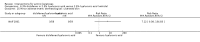






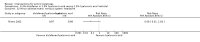



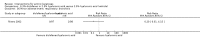


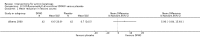

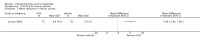

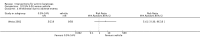








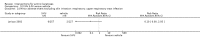
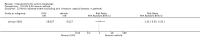
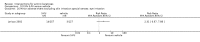












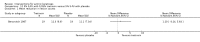



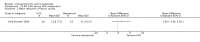










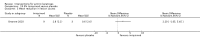

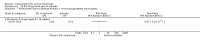








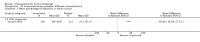





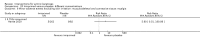















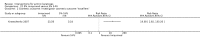

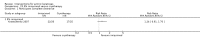










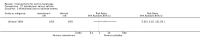














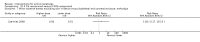
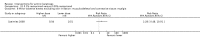
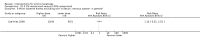
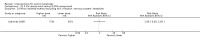







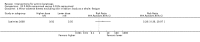
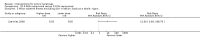

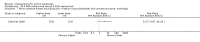
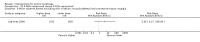
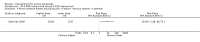
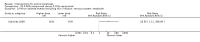
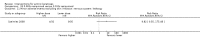
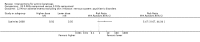
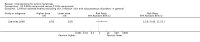



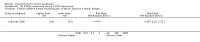
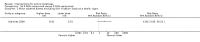

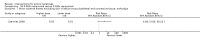






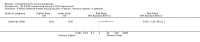
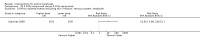
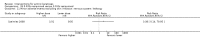
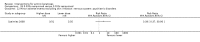
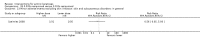


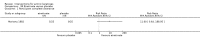
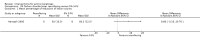

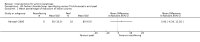
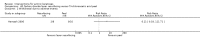




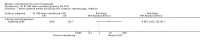




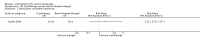
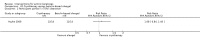

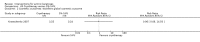
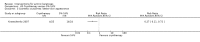
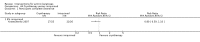
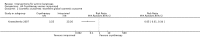


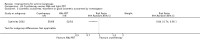
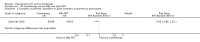













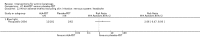

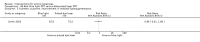









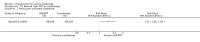





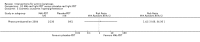





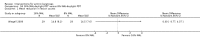
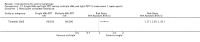
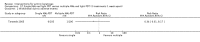


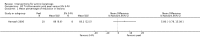

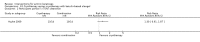


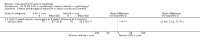




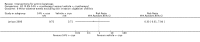

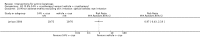


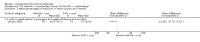


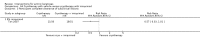





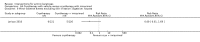

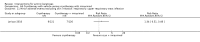
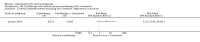



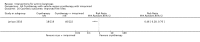
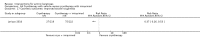






Update of
- doi: 10.1002/14651858.CD004415
Similar articles
-
Interventions for cutaneous Bowen's disease.Cochrane Database Syst Rev. 2013 Jun 24;2013(6):CD007281. doi: 10.1002/14651858.CD007281.pub2. Cochrane Database Syst Rev. 2013. PMID: 23794286 Free PMC article.
-
Light therapies for acne.Cochrane Database Syst Rev. 2016 Sep 27;9(9):CD007917. doi: 10.1002/14651858.CD007917.pub2. Cochrane Database Syst Rev. 2016. PMID: 27670126 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Topical treatments for cutaneous warts.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD001781. doi: 10.1002/14651858.CD001781.pub3. Cochrane Database Syst Rev. 2012. PMID: 22972052 Free PMC article.
Cited by
-
Ingenol mebutate in the treatment of actinic keratoses: clearance rate and adverse effects.An Bras Dermatol. 2018 Jul-Aug;93(4):529-534. doi: 10.1590/abd1806-4841.20186982. An Bras Dermatol. 2018. PMID: 30066759 Free PMC article. Clinical Trial.
-
Advancements in elucidating the pathogenesis of actinic keratosis: present state and future prospects.Front Med (Lausanne). 2024 Mar 19;11:1330491. doi: 10.3389/fmed.2024.1330491. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38566927 Free PMC article. Review.
-
[Actinic keratosis: New concept and therapeutic update].Aten Primaria. 2017 Oct;49(8):492-497. doi: 10.1016/j.aprim.2017.01.004. Epub 2017 Apr 17. Aten Primaria. 2017. PMID: 28427916 Free PMC article. Spanish.
-
Updates on Psoriasis and Cutaneous Oncology: Proceedings from the 2015 MauiDerm Meeting.J Clin Aesthet Dermatol. 2015 Sep;8(9 Suppl):S4-S26. J Clin Aesthet Dermatol. 2015. PMID: 26504503 Free PMC article. No abstract available.
-
Economic Evaluation of Tirbanibulin for the Treatment of Actinic Keratosis in Scotland.Pharmacoecon Open. 2023 May;7(3):443-454. doi: 10.1007/s41669-023-00410-5. Epub 2023 Apr 3. Pharmacoecon Open. 2023. PMID: 37012513 Free PMC article.
References
References to studies included in this review
Akar 2001 {published data only}
-
- Akar A, Tastan HB, Erbil H, Arca E, Kurumlu Z, Gur AR. Efficacy and safety assessment of 0.5% and 1% colchicine cream in the treatment of actinic keratoses. Journal of Dermatological Treatment 2001;12(4):199‐203. [PUBMED: 12241628] - PubMed
Alberts 2000 {published data only}
-
- Alberts DS, Dorr RT, Einspahr JG, Aickin M, Saboda K, Xu MJ, et al. Chemoprevention of human actinic keratoses by topical 2‐(difluoromethyl)‐dl‐ornithine. Cancer Epidemiology, Biomarkers & Prevention 2000;9(12):1281‐6. [PUBMED: 11142412] - PubMed
-
- Einspahr JG, Nelson MA, Saboda K, Warneke J, Bowden GT, Alberts DS. Modulation of biologic endpoints by topical difluoromethylornithine (DFMO), in subjects at high‐risk for nonmelanoma skin cancer. Clinical Cancer Research 2002;8(1):149‐55. [PUBMED: 11801552 ] - PubMed
Alirezai 1994 {published data only}
-
- Alirezai M, Dupuy P, Amblard P, Kalis B, Souteyrand P, Frappaz A, et al. Clinical evaluation of topical isotretinoin in the treatment of actinic keratoses. Journal of the American Academy of Dermatology 1994;30(3):447‐51. [PUBMED: 8113458] - PubMed
Alomar 2007 {published data only}
-
- Alomar A, Bichel J, McRae S. Vehicle‐controlled, randomized, double‐blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. British Journal of Dermatology 2007;157(1):133‐41. [PUBMED: 17501955 ] - PubMed
Anderson 2009 {published data only}
-
- Anderson L, Schmieder GJ, Werschler WP, Tschen EH, Ling MR, Stough DB, et al. Randomized, double‐blind, double‐dummy, vehicle controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. Journal of the American Academy of Dermatology 2009;60(6):934‐43. [PUBMED: 19467365 ] - PubMed
-
- NCT00375739. Study to Determine the Safety of PEP005 0.025% and 0.05% Topical Gel in Patients With Actinic Keratoses. clinicaltrials.gov/ct2/show/NCT00375739 (accessed 14 March 2011).
Bercovitch 1987 {published data only}
-
- Bercovitch L. Topical chemotherapy of actinic keratoses of the upper extremity with tretinoin and 5‐fluorouracil: a double‐blind controlled study. British Journal of Dermatology 1987;116(4):549‐52. [PUBMED: 3555597] - PubMed
Chen 2003 {published data only}
-
- Chen K, Yap LM, Marks R, Shumack S. Short‐course therapy imiquimod 5% cream for solar keratoses: A randomized controlled trial. Australasian Journal of Dermatology 2003;44(4):250‐5. [PUBMED: 14616490] - PubMed
Dragieva 2004a {published and unpublished data}
-
- Dragieva G, Prinz B, Hafner J, Dummer R, Burg G, Binswanger U, et al. Topical photodynamic therapy with MAL in the treatment of actinic keratoses in transplant recipients. [Abstract PD‐16] The 85th BAD Annual Meeting 5‐8th July 2005, Glasgow, UK. British Journal of Dermatology 2005;153(Suppl 1):97.
-
- Dregieva G, Prinz B, Hafner J, Dummer R, Burg G, Binswanger U, et al. A randomized controlled clinical trial of topical photodynamic therapy with methyl aminolaevulinate in the treatment of actinic keratoses in transplant recipients. British Journal of Dermatology 2004;151(1):196‐200. [PUBMED: 15270891] - PubMed
Fariba 2006 {published data only}
-
- Fariba I, Ali A, Hossein SA, Atefeh S, Atarzadeh Behbahan SA. Efficacy of 3% diclofenac gel for the treatment of actinic keratoses: a randomized, double‐blind, placebo controlled study. Indian Journal of Dermatology, Venereology & Leprology 2006;72(5):346‐9. [PUBMED: 17050927] - PubMed
Foote 2009 {published data only}
Freeman 2003 {published and unpublished data}
-
- Foley P, Freeman M, Vinciullo C, Spelman L, Murrell D, Weightman W, et al. Photodynamic therapy using methyl aminolaevulinate with cryotherapy in actinic keratosis: an Australian study. [Abstract P‐31] The 85th BAD Annual Meeting 5‐8th July 2005, Glasgow, UK. British Journal of Dermatology 2005; Vol. 153, issue Suppl 1:29.
-
- Freeman M, Vinciullo C, Francis D, Spelman L, Nguyen R, Fergin P, et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: a prospective, randomized study. Journal of Dermatological Treatment 2003;14(2):99‐106. [PUBMED: 12775317] - PubMed
Gebauer 2003 {published data only}
-
- Gebauer K, Brown P, Varigos G. Topical diclofenac in hyaluronan gel for the treatment of solar keratoses. Australasian Journal of Dermatology 2003;44(1):40‐3. [PUBMED: 12581080 ] - PubMed
-
- Paquet M. Confirmation of ITT analysis for efficacy [personal communication]. Email to: Dr Gebauer 27 March 2011.
Gebauer 2009 {published data only}
-
- Gebauer K, Shumack S, Cowen P S. Effect of dosing frequency on the safety and efficacy of imiquimod 5% cream for treatment of actinic keratosis on the forearms and hands: a phase II, randomized placebo‐controlled trial. British Journal of Dermatology 2009;161(4):897‐903. [PUBMED: 19545297] - PubMed
Hanke 2010 {published and unpublished data}
-
- Hanke CW, Beer KR, Stockfleth E, Wu J, Rosen T, Levy S. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo‐controlled studies of daily application to the face and balding scalp for two 3‐week cycles. Journal of the American Academy of Dermatology 2010;62(4):573‐81. [PUBMED: 20133012] - PubMed
-
- NCT00603798. Safety and Effectiveness Study of Imiquimod Creams for the Treatment of Actinic Keratoses (actinic keratoses). clinicaltrials.gov/ct2/show/NCT00603798 (accessed 14 March 2011).
Hantash 2006 {published data only}
-
- Hantash BM, Stewart DB, Cooper ZA, Rehmus WE, Koch RJ, Swetter SM. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Archives of Dermatology 2006;142(8):976‐82. [PUBMED: 16924046 ] - PubMed
Hauschild 2009a {published data only}
-
- Hauschild A, Stockfleth E, Popp G, Borrosch F, Bruning H, Dominicus R, et al. Optimization of photodynamic therapy with a novel self‐adhesive 5‐aminolaevulinic acid patch: results of two randomized controlled phase III studies. British Journal of Dermatology 2009;160(5):1066‐74. [PUBMED: 19222455] - PubMed
-
- NCT00308854. PD P 506 A‐PDT Versus Placebo‐PDT for the treatment of actinic keratose. clinicaltrials.gov/ct2/show/NCT00308854 (accessed 14 March 2011).
Hauschild 2009b {published data only}
-
- Hauschild A, Stockfleth E, Popp G, Borrosch F, Bruning H, Dominicus R, et al. Optimization of photodynamic therapy with a novel self‐adhesive 5‐aminolaevulinic acid patch: results of two randomized controlled phase III studies. British Journal of Dermatology 2009;160(5):1066‐74. [PUBMED: 19222455] - PubMed
-
- NCT00308867. PD P 506 A‐PDT Versus Placebo‐PDT and Cryosurgery for the Treatment of actinic keratose. clinicaltrials.gov/ct2/show/NCT00308867 (accessed 14 March 2011).
Hauschild 2009c {published data only}
-
- Hauschild A, Popp G, Stockfleth E, Meyer K G, Imberger D, Mohr P, et al. Effective photodynamic therapy of actinic keratoses on the head and face with a novel, self‐adhesive 5‐aminolaevulinic acid patch. Experimental Dermatology 2009;18(2):116‐21. [PUBMED: 18643849] - PubMed
Huyke 2009 {published data only}
-
- Huyke C, Reuter J, Rodig M, Kersten A, Laszczyk M, Scheffler A, et al. Treatment of actinic keratoses with a novel betulin‐based oleogel. A prospective, randomized, active‐controlled pilot study. Journal Der Deutschen Dermatologischen Gesellschaft 2009;7(2):128‐133. [PUBMED: 18808378] - PubMed
Jeffes 2001 {published data only}
-
- Jeffes E, McCullough JL, Weinstein GD, Kaplan R, Glazer SD, Taylor JR. Photodynamic therapy of actinic keratoses with topical aminolevulinic acid hydrochloride and fluorescent blue light. Journal of the American Academy of Dermatology 2001;45(1):96‐104. [PUBMED: 11423841] - PubMed
Jorizzo 2002 {published and unpublished data}
-
- Dermik Laboratories. Carac product insert. //products.sanofi.us/carac/carac.html (accessed 24 March 2011).
-
- Jorizzo J. Topical treatment of actinic keratosis with fluorouracil: is irritation associated with efficacy?. Journal of Drugs in Dermatology 2004;3(1):21‐6. [PUBMED: 14964743] - PubMed
-
- Jorizzo J, Stewart D, Bucko A, Davis SA, Espy P, Hino P, et al. Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1‐, 2‐, or 4‐week treatment in patients with actinic keratosis. Cutis 2002;70(6):335‐9. [PUBMED: 12502122] - PubMed
Jorizzo 2004 {published data only}
-
- Jorizzo J, Weiss J, Furst K, VandePol C, Levy S. Effect of a 1‐week treatment with 0.5% topical fluorouracil on occurrence of actinic keratosis after cryosurgery: A randomized, vehicle‐controlled clinical trial. Archives of Dermatology 2004;140:813‐6. [PUBMED: 15262691] - PubMed
Jorizzo 2006 {published data only}
-
- Jorizzo J, Weiss J, Vamvakias G. One‐week treatment with 0.5% fluorouracil cream prior to cryosurgery in patients with actinic keratoses: a double‐blind, vehicle‐controlled, long‐term study. Journal of Drugs in Dermatology 2006;5(2):133‐9. [PUBMED: 16485881 ] - PubMed
Jorizzo 2007 {published data only}
-
- Jorizzo J, Dinehart S, Matheson R, Moore JK, Ling M, Fox TL, et al. Vehicle‐controlled, double‐blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. Journal of the American Academy of Dermatology 2007;57(2):265‐8. [PUBMED: 17512087] - PubMed
Jorizzo 2010 {published and unpublished data}
-
- Jorizzo JL, Markowitz O, Lebwohl MG, Bourcier M, Kulp J, Meng TC, et al. A randomized, double‐blinded, placebo‐controlled, multicenter, efficacy and safety study of 3.75% imiquimod cream following cryosurgery for the treatment of actinic keratoses. Journal of Drugs in Dermatology 2010;9(9):1101‐8. [PUBMED: 20865842] - PubMed
-
- Lee J, Jorizzo J, Lebwohl M, Markowitz O, Levy S. Cryosurgery followed by imiquimod 3.75% to treat actinic keratosis. 69th Annual Meeting of the American Academy of Dermatology New Orleans, LA United States, February 2011. Journal of the American Academy of Dermatology 2011; Vol. 64, issue 2 Suppl 1:AB2.
-
- NCT00894647. Safety and effectiveness study of actinic keratosis treatment following cryosurgery. clinicaltrials.gov/ct2/show/NCT00894647 (accessed 14 March 2011).
Kang 2003 {published data only}
-
- Kang S, Goldfarb MT, Weiss JS, Metz RD, Hamilton TA, Voorhees JJ, et al. Assessment of adapalene gel for the treatment of actinic keratoses and lentigines: a randomized trial. Journal of the American Academy of Dermatology 2003;49(1):83‐90. [PUBMED: 12833014 ] - PubMed
Kaufmann 2008 {published data only}
-
- Kaufmann R, Spelman L, Weightman W, Reifenberger J, Szeimies R M, Verhaeghe E, et al. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate‐photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. British Journal of Dermatology 2008;158(5):994‐9. [PUBMED: 18341663] - PubMed
Korman 2005 {published data only}
-
- Korman N, Moy R, Ling M, Matheson R, Smith S, McKane S, et al. Dosing with 5% imiquimod cream 3 times per week for the treatment of actinic keratosis: Results of two phase 3, randomized, double‐blind, parallel‐group, vehicle‐controlled trials. Archives of Dermatology 2005;141(4):467‐73. [PUBMED: 15837864 ] - PubMed
Kose 2008 {published data only}
-
- Kose O, Koc E, Erbil AH, Caliskan E, Kurumlu Z. Comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% imiquimod cream in the treatment of actinic keratosis. Journal of Dermatological Treatment 2008;19(3):159‐63. [PUBMED: 18569272 ] - PubMed
Krawtchenko 2007 {published data only}
-
- Krawtchenko N, Roewert‐Huber J, Ulrich M, Mann I, Sterry W, Stockfleth E. A randomized study of topical 5% imiquimod vs. topical 5‐fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1‐year follow‐up. British Journal of Dermatology 2007;157(Suppl 2):34‐40. [PUBMED: 18067630] - PubMed
Kulp‐Shorten 1993 {published data only}
-
- Kulp‐Shorten C, Konnikov N, Callen J. Comparative Evaluation of the efficacy and Safety of Masoprocol and 5‐Fluorouracil Cream for the Treatment of Multiple Actinic Keratoses of the Head and Neck. Journal of Geriatric Dermatology 1993;1(4):161‐8.
Lebwohl 2004 {published and unpublished data}
-
- Graceway Pharmaceuticals. Aldara product monograph. //www.gracewaypharma.com/sites/default/files/products/aldara_ppi.pdf (accessed 24 March 2011).
-
- Lebwohl M, Dinehart S, Whiting D, Lee PK, Tawfik N, Jorizzo J, et al. Imiquimod 5% cream for the treatment of actinic keratosis: results from two phase III, randomized, double‐blind, parallel group, vehicle‐controlled trials. Journal of the American Academy of Dermatology 2004;50(5):714‐21. [PUBMED: 15097955] - PubMed
Loven 2002 {published data only}
-
- Loven K, Stein L, Furst K, Levy S. Evaluation of the efficacy and tolerability of 0.5% fluorouracil cream and 5% fluorouracil cream applied to each side of the face in patients with actinic keratosis. Clinical Therapeutics 2002;24(6):990‐1000. [PUBMED: 12117087] - PubMed
McEwan 1997 {published data only}
-
- McEwan LE, Smith JG. Topical diclofenac/hyaluronic acid gel in the treatment of solar keratoses. Australasian Journal of Dermatology 1997;38(4):187‐9. [PUBMED: 9431711] - PubMed
Misiewicz 1991 {published data only}
-
- Misiewicz J, Sendagorta E, Golebiowska A, Lorenc B, Czarnetzki BM, Jablonska S. Topical treatment of multiple actinic keratoses of the face with aretinoid methyl sulfone (Ro 14‐9706) cream versus tretinoin cream: a double‐blind, active‐controlled study. Journal of the American Academy Dermatology 1991;24(3):448‐51. [PUBMED: 2061443] - PubMed
Moloney 2007 {published data only}
-
- Moloney FJ, Collins P. Randomized, double‐blind, prospective study to compare topical 5‐aminolaevulinic acid methylester with topical 5‐aminolaevulinic acid photodynamic therapy for extensive scalp actinic keratosis. British Journal of Dermatology 2007;157(1):87‐91. [PUBMED: 17501954 ] - PubMed
Moloney 2010 {published data only}
-
- ACTRN12609000490279. Randomized, double‐blind, placebo controlled study to assess efficacy of topical nicotinamide in the treatment and prevention of actinic keratosis.. anzctr.org.au/trial_view.aspx?ID=83879 (accessed 14 March 2011).
-
- Moloney F, Vestergaard M, Radojkovic B, Damian D. Randomized, double‐blinded, placebo controlled study to assess the effect of topical 1% nicotinamide on actinic keratoses. British Journal of Dermatology 2010; Vol. 162, issue 5:1138‐9. [PUBMED: 20199551] - PubMed
Moriarty 1982 {published data only}
-
- Moriarty M, Dunn J, Darragh A, Lambe R, Brick I. Etretinate in treatment of actinic keratosis: a double‐blind crossover study. Lancet 1982;1(8268):364‐5. [PUBMED: 6120350] - PubMed
Morton 2006 {published data only}
-
- Morton C, Campbell S, Gupta G, Keohane S, Lear J, Zaki I, et al. Intraparticipant, right‐left comparison of topical methyl aminolaevulinate‐photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized controlled study. British Journal of Dermatology 2006;155(5):1029‐36. [PUBMED: 17034536] - PubMed
NCT00774787 {unpublished data only}
-
- NCT00774787. Topical imiquimod cream in combination with cryotherapy for the treatment of actinic keratoses. http://www.clinicaltrials.gov/ct2/show/NCT00774787 (accessed 14 March 2011).
NCT00828568 Aldara {unpublished data only}
-
- NCT00828568. A Therapeutic Equivalence Study of Two Imiquimod Cream 5% Treatments for Patients With Actinic Keratosis. http://www.clinicaltrials.gov/ct2/show/NCT00828568 (accesssed 14 March 2011).
NCT00828568 Taro {unpublished data only}
-
- NCT00828568. A Therapeutic Equivalence Study of Two Imiquimod Cream 5% Treatments for Patients With Actinic Keratosis. clinicaltrials.gov/ct2/show/NCT00828568 (accessed 14 March 2011).
Olsen 1991 {published data only}
-
- Olsen EA, Abernethy ML, Kulp‐Shorten C, Callen JP, Glazer SD, Huntley A, et al. A double‐blind, vehicle‐controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. Journal of the American Academy of Dermatology 1991;24(5 Pt 1):738‐43. [PUBMED: 1869646] - PubMed
Ooi 2006 {published data only}
-
- Ooi T, Barnetson RS, Zhuang L, McKane S, Lee JH, Slade HB, et al. Imiquimod‐induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. British Journal of Dermatology 2006;154(1):72‐8. [PUBMED: 16403097] - PubMed
Ortonne 2010 {published data only}
-
- NCT00294320. Evaluation of Two Different Non‐Invasive Techniques to Monitor the Clearance of Actinic Keratosis Lesions. clinicaltrials.gov/ct2/show/NCT00294320 (accessed 14 March 2011).
-
- Ortonne JP, Gupta G, Ortonne N, Duteil L, Queille C, Mallefet P. Effectiveness of cross polarized light and fluorescence diagnosis for detection of sub‐clinical and clinical actinic keratosis during imiquimod treatment. Experimental Dermatology 2010;19(7):641‐7. [PUBMED: 20201959] - PubMed
Ostertag 2006 {published data only}
-
- Ostertag JU, Quaedvlieg PJF, Geer S, Nelemans P, Christianen ME, Neumann MH, et al. A clinical comparison and long‐term follow‐up of topical 5‐fluorouracil versus laser resurfacing in the treatment of widespread actinic keratoses. Lasers in Surgery & Medicine 2006; Vol. 38, issue 8:731‐9. [PUBMED: 16912977] - PubMed
Pariser 2003 {published data only}
-
- Pariser DM, Lowe NJ, Stewart DM, Jarratt MT, Lucky AW, Pariser RJ, et al. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: results of a prospective randomized multicenter trial. Journal of the American Academy of Dermatology 2003;48(2):227‐32. [PUBMED: 12582393] - PubMed
Pariser 2008 {published and unpublished data}
-
- Galderma. Metvixia product label. http://metvixia.com/pi/MetvixiaPI.pdf (accessed on 26 April 2011).
-
- NCT00306800. Metvix PDT Versus Vehicle PDT With Aktilite CL128 Lamp in Patients With Actinic Keratosis on the Face and Scalp. clinicaltrials.gov/ct2/show/NCT00306800 (accessed 14 March 2011). [NCT00306800]
-
- Pariser D, Loss R, Jarratt M, Abramovits W, Spencer J, Geronemus R, et al. Topical methyl‐aminolevulinate photodynamic therapy using red light‐emitting diode light for treatment of multiple actinic keratoses: a randomized, double‐blind, placebo‐controlled study. Journal of the American Academy of Dermatology 2008;59(4):569‐76. [PUBMED: 18707799 ] - PubMed
Perrett 2007 {published data only}
-
- Perrett CM, McGregor JM, Warwick J, Karran P, Leigh IM, Proby CM, et al. Treatment of post‐transplant premalignant skin disease: a randomized intrapatient active‐controlled study of 5‐fluorouracil cream and topical photodynamic therapy. British Journal of Dermatology 2007;156(2):320‐8. [PUBMED: 17223873 ] - PMC - PubMed
Persaud 2002 {published data only}
-
- Persaud AN, Shamuelova E, Sherer D, Lou W, Singer G, Cervera C, et al. Clinical effect of imiquimod 5% cream in the treatment of actinic keratosis. Journal of the American Academy of Dermatology 2002;47(4):553‐6. [PUBMED: 12271300] - PubMed
Photocure‐Australian 2004 {unpublished data only}
-
- Photocure ASA. Metvixia cream, 16.8%, product insert. Data on file 2004.
Photocure‐US 2004 {unpublished data only}
-
- Photocure ASA. Metvixia cream 16.8%, product insert. Data on file 2004.
Piacquadio 2004 {published and unpublished data}
-
- DUSA. Levulan Kerastick (aminolevulinic acid HCl) for topical solution, 20%. Product monograph. accessdata.fda.gov/drugsatfda_docs/label/2010/020965s007lbl.pdf (accessed 5 April 2011).
-
- Fowler JF, Zax RH. Aminolevulinic acid hydrochloride with photodynamic therapy: efficacy outcomes and recurrence 4 years after treatment. Cutis 2002;69(6 Suppl):2‐7. [PUBMED: 12095067 ] - PubMed
-
- Piacquadio DJ, Chen DM, Farber HF, Fowler JF, Glazer SD, Goodman JJ, et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator‐blinded, phase 3, multicenter trials. Archives of Dermatology 2004;140(1):41‐6. [PUBMED: 14732659] - PubMed
Rivers 2002 {published and unpublished data}
-
- Solaraze gel: Diclofenac Sodium 3% ‐ package insert. www.solaraze.com/solaraze/pdf/solaraze_pi.pdf (accessed January 2012).
-
- Rivers JK, Arlette J, Shear N, Guenther L, Carey W, Poulin Y. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronoan gel. British Journal of Dermatology 2002;146(1):94‐100. [PUBMED: 11841372] - PubMed
Seckin 2009 {published data only}
-
- Seckin D, Cerman AA, Yildiz A, Ergun T. Can topical calcipotriol be a treatment alternative in actinic keratoses? A preliminary report. Journal of Drugs in Dermatology 2009;8(5):451‐4. [PUBMED: 19537367] - PubMed
Shaffelburg 2009 {published data only}
-
- Shaffelburg M. Treatment of actinic keratoses with sequential use of photodynamic therapy; and imiquimod 5% cream. Journal of Drugs in Dermatology 2009;8(1):35‐9. [PUBMED: 19180894] - PubMed
Siller 2009 {published data only}
-
- NCT00107965. Study to Determine the Safety of Two Applications of PEP005 Topical Gel to Actinic Keratoses. clinicaltrials.gov/ct2/show/NCT00107965 (accessed 14 March 2011).
-
- Siller G, Gebauer K, Welburn P, Katsamas J, Ogbourne SM. PEP005 (ingenol mebutate) gel, a novel agent for the treatment of actinic keratosis: results of a randomized, double‐blind, vehicle‐controlled, multicentre, phase IIa study. Australasian Journal of Dermatology 2009;50(1):16‐22. [PUBMED: 19178487 ] - PubMed
Smith 2003 {published data only}
-
- Smith S, Piacquadio D, Morhenn V, Atkin D, Fitzpatrick R. Short incubation PDT versus 5‐FU in treating actinic keratosis. Journal of Drugs in Dermatology 2003;2(6):629‐35. [PUBMED: 14711141] - PubMed
Solaraze study 2 {unpublished data only}
-
- Solaraze gel: Diclofenac Sodium 3% ‐ package insert. https://www.solaraze.com/solaraze/pdf/solaraze_pi.pdf (accessed 24 March 2011).
Sotiriou 2009 {published data only}
-
- Sotiriou E, Apalla Z, Maliamani F, Zaparas N, Panagiotidou D, Ioannides D. Intraparticipant, right‐left comparison of topical 5‐aminolevulinic acid photodynamic therapy vs. 5% imiquimod cream for actinic keratoses on the upper extremities. Journal of the European Academy of Dermatology & Venereology 2009;23(9):1061‐5. [PUBMED: 19470041 ] - PubMed
Stockfleth 2002 {published data only}
-
- Stockfleth E, Meyer T, Benninghoff B, Salasche S, Paradoulos L, Ulrich C, et al. A randomized, double‐blind, vehicle‐controlled study to asses 5% imiquimod cream for the treatment of multiple actinic keratoses. Archives of Dermatology 2002;138(11):1498‐502. [PUBMED: 12437457] - PubMed
Swanson 2010a {published and unpublished data}
-
- Graceway Pharmaceuticals. Zyclara product monograph. http://www.gracewaypharma.com/sites/default/files/products/Zyclara%20Ful... (accessed 26 April 2011).
-
- NCT00605176. Safety and Effectiveness Study of Imiquimod Creams for Treatment of Actinic Keratoses (actinic keratoses). //clinicaltrials.gov/ct2/show/NCT00605176 (accessed 14 March 2011).
-
- Swanson N, Abramovits W, Berman B, Kulp J, Rigel DS, Levy S. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo‐controlled studies of daily application to the face and balding scalp for two 2‐week cycles. Journal of the American Academy of Dermatology 2010;62(4):582‐90. [PUBMED: 20133013] - PubMed
Swanson 2010b {published and unpublished data}
-
- Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman B. Ingenol mebutate gel for actinic keratosis. New England Journal of Medicine 2012;366(11):1010‐19. [PUBMED: 22417254] - PubMed
-
- NCT00742391. A Multicenter Study to Evaluate the Efficacy and Safety of PEP005 (Ingenol Mebutate) Gel When Used to Treat Actinic Keratoses (actinic keratoses) on the Non Head Locations. //clinicaltrials.gov/ct2/show/NCT00742391 (accessed 14 March 2011).
-
- Swanson N. Multicenter, randomized, parallel‐group, double‐blind, vehicle‐controlled study to evaluate the efficacy and safety of PEP005 (ingenol mebutate) gel, 0.05% in patients with actinic keratoses on nonhead locations. Journal of the American Academy of Dermatology 2010; Vol. 62, issue 3 Suppl 1:AB2.
Szeimies 2002 {published data only}
-
- Szeimies RM, Karrer S, Radakovic‐Fijan S, Tanew A, Calzavara‐Pinton PG, Zane C, et al. Photodynamic therapy using topical methyl 5‐aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. Journal of the American Academy of Dermatology 2002;47(2):258‐62. [PUBMED: 12140473] - PubMed
Szeimies 2004 {published data only}
-
- Szeimies RM, Gerritsen MJ, Gupta G, Ortonne JP, Serresi S, Bichel J, et al. Imiquimod 5% cream for the treatment of actinic keratosis: Results from a phase III, randomized, double‐blind, vehicle‐controlled, clinical trial with histology. Journal of the American Academy Dermatology 2004;51(4):547‐55. [PUBMED: 15389189] - PubMed
Szeimies 2008 {published data only}
-
- Szeimies RM, Bichel J, Ortonne JP, Stockfleth E, Lee J, Meng TC. A phase II dose‐ranging study of topical resiquimod to treat actinic keratosis. British Journal of Dermatology 2008;159(1):205‐10. [PUBMED: 18476957] - PubMed
Szeimies 2009 {published and unpublished data}
-
- Galderma. Metvixia product label. http://metvixia.com/pi/MetvixiaPI.pdf (accessed 26 April 2011).
-
- NCT00304239. Metvix PDT Versus Vehicle PDT With Aktilite CL128 Lamp in Patients With Actinic Keratosis on the Face and Scalp. clinicaltrials.gov/ct2/show/NCT00304239 (accessed 14 March 2011).
-
- Szeimies R, Matheson RT, Davis SA, Bhatia AC, Frambach Y, Klovekorn W, et al. Topical methyl aminolevulinate photodynamic therapy using red light‐emitting diode light for multiple actinic keratoses: a randomized study. Dermatologic Surgery 2009;35(4):586‐592. [PUBMED: 19309347] - PubMed
Szeimies 2010b {published data only}
-
- Szeimies RM, Radny P, Sebastian M, Borrosch F, Dirschka T, Krahn‐Senftleben G, et al. Photodynamic therapy with BF‐200 ALA for the treatment of actinic keratosis: results of a prospective, randomized, double‐blind, placebo‐controlled phase III study. British Journal of Dermatology 2010;163(2):386‐94. [PUBMED: 20518784] - PubMed
Tan 2007 {published data only}
-
- NCT00110682. Study of Imiquimod 5% Cream in Addition to Cryotherapy in the Management of Actinic Keratoses. http://clinicaltrials.gov/ct2/show/NCT00110682 (accessed 14 March 2011).
-
- Tan JK, Thomas DR, Poulin Y, Maddin F, Tang J. Efficacy of imiquimod as an adjunct to cryotherapy for actinic keratoses. Journal of Cutaneous Medicine and Surgery 2007;11(5):195‐201. [PUBMED: 18042331] - PubMed
Tanghetti 2007 {published and unpublished data}
-
- Paquet M. Tanghetti and Werschler 2007. Email to: E A Tanghetti 12 April 2011.
-
- Tanghetti E, Werschler P. Comparison of 5% 5‐fluorouracil cream and 5% imiquimod cream in the management of actinic keratoses on the face. Journal of Drugs in Dermatology 2007;6(2):144‐7. [PUBMED: 17373172] - PubMed
Tarstedt 2005 {published and unpublished data}
-
- Tarstedt M, Rosdahl I, Berne B, Svanberg K, Wennberg AM. A Randomized Multicenter Study to Compare Two Treatment Regimens of Topical Methyl Aminolevulinate (Metvix)‐PDT in Actinic Keratosis of the Face and Scalp. Acta Dermato‐Venereologica 2005;85(5):424‐428. [PUBMED: 16159735] - PubMed
-
- Tarstedt M, Wennberg A‐M, Rosdahl I, Persson BE, Berne B, Bojs G, et al. A comparison of two treatment regimes using photodynamic therapy with Metvix in actinic keratosis [Abstract FC8‐3] [The 12th Congress of the European Academy of Dermatology and Venerology. Barcelona, Spain 15‐18th October 2003]. Journal of the European Academy of Dermatology & Venerology 2003;17(Suppl 3):126.
Thompson 1993 {published data only}
-
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. New England Journal of Medicine 1993;329(16):1147‐51. [PUBMED: 8377777] - PubMed
Tong 1996 {published data only}
-
- Tong DW, Barnetson RS. Beta‐1,3‐D‐glucan gel in the treatment of solar keratoses. Australasian Journal of Dermatology 1996;37(3):137‐8. [PUBMED: 8771866] - PubMed
Ulrich 2007 {published data only}
-
- NCT00189267. A Study to Evaluate the Effectiveness and Safety of Multiple Applications of Imiquimod 5% Cream for the Treatment of Actinic Keratoses in Organ Transplant Recipients. clinicaltrials.gov/ct2/show/NCT00189267 (accessed 14 March 2011).
-
- Ulrich C, Bichel J, Euvrard S, Guidi B, Proby CM, Kerkhof PC, et al. Topical immunomodulation under systemic immunosuppression: results of a multicentre, randomized, placebo‐controlled safety and efficacy study of imiquimod 5% cream for the treatment of actinic keratoses in kidney, heart, and liver transplant patients. British Journal of Dermatology 2007;157(Suppl 2):25‐31. [PUBMED: 18067628] - PMC - PubMed
Ulrich 2010 {published and unpublished data}
-
- Ulrich C. The efficacy and safety of diclofenac 3% gel treatment of multiple actinic keratoses in organ transplant patients: A double‐blind, randomized, vehicle‐controlled study. Journal of the American Academy of Dermatology 2010; Vol. 62, issue 3 Suppl 1:AB107.
-
- Ulrich C, Johannsen A, Rowert‐Huber J, Ulrich M, Sterry W, Stockfleth E. Results of a randomized, placebo‐controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. European Journal of Dermatology 2010;20(4):482‐8. [PUBMED: 20507841] - PubMed
Van der Geer 2009 {published data only}
-
- Geer S, Krekels GA. Treatment of actinic keratoses on the dorsum of the hands: ALA‐PDT versus diclofenac 3% gel followed by ALA‐PDT. A placebo‐controlled, double‐blind, pilot study. Journal of Dermatological Treatment 2009;20(5):259‐65. [PUBMED: 19370466] - PubMed
von Felbert 2010 {published data only}
-
- Felbert V, Hoffmann G, Hoff‐Lesch S, Abuzahra F, Renn C N, Braathen L R, et al. Photodynamic therapy of multiple actinic keratoses: reduced pain through use of visible light plus water‐filtered infrared A compared with light from light‐emitting diodes. British Journal of Dermatology 2010;163(3):607‐15. [PUBMED: 20426780] - PubMed
Weiss 2002 {published and unpublished data}
-
- Carac® Cream, 0.5% ‐ product insert. http://products.sanofi.us/carac/carac.html (accessed January 2012).
-
- Jorizzo J. Topical treatment of actinic keratosis with fluorouracil: is irritation associated with efficacy?. Journal of Drugs in Dermatology 2004;3(1):21‐6. [PUBMED: 14964743] - PubMed
-
- Weiss J, Menter A, Hevia O, Jones T, Ling M, Rist T, et al. Effective Treatment of Actinic Keratosis With 0.5% Fluorouracil Cream for 1, 2 or 4 Weeks. Cutis 2002;70(2 Suppl):22‐9. [PUBMED: 12353677] - PubMed
Wiegell 2008 {published data only}
-
- NCT00432224. Treatment of Actinic Keratoses With Photodynamic Therapy Using Sunlight Versus Red Light. clinicaltrials.gov/ct2/show/NCT00432224 (accessed 14 March 2011).
-
- Wiegell SR, Haedersdal M, Philipsen PA, Eriksen P, Enk CD, Wulf HC. Continuous activation of PpIX by daylight is as effective as and less painful than conventional photodynamic therapy for actinic keratoses; a randomized, controlled, single‐blinded study. British Journal of Dermatology 2008;158(4):740‐6. [PUBMED: 18294318] - PubMed
Wiegell 2009 {published data only}
-
- Wiegell SR, Haedersdal M, Eriksen P, Wulf HC. Photodynamic therapy of actinic keratoses with 8% and 16% methyl aminolaevulinate and home‐based daylight exposure: a double‐blinded randomized clinical trial. British Journal of Dermatology 2009;160(6):1308‐14. [PUBMED: 19416257] - PubMed
-
- Wiegell SR, Hædersdal M, Wulf H. Photodynamic therapy of actinic keratoses with two different concentrations of methylaminolevulinate and home‐based daylight exposure – a randomized, controlled, double blinded study. Journal of Investigative Dermatology 2008;128(S1):S204.
Wiegell 2011a {published data only}
-
- NCT00711178. A Randomized Multicenter Study of Daylight Mediated Photodynamic Therapy (PDT). clinicaltrials.gov/ct2/show/NCT00711178 (accessed 14 March 2011).
-
- Wiegell SR, Fabricius S, Stender IM, Berne B, Kroon S, Andersen BL, et al. A randomized, multicentre study of directed daylight exposure times of 1½ vs. 2½h in daylight‐mediated photodynamic therapy with methyl aminolaevulinate in patients with multiple thin actinic keratoses of the face and scalp.. British Journal of Dermatology 2011;164(5):1083‐90. [PUBMED: 21219287] - PubMed
Wolf 2001 {published and unpublished data}
-
- Solaraze gel: Diclofenac Sodium 3% ‐ package insert. Doak Dermatologics.
-
- Wolf JE, Taylor JR, Tschen E, Kang S. Topical 3.0% diclofenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. International Journal of Dermatology 2001;40(11):709‐13. [PUBMED: 11737438] - PubMed
Zeichner 2009 {published data only}
-
- Zeichner JA, Stern DW, Uliasz A, Itenberg S, Lebwohl M. Placebo‐controlled, double‐blind, randomized pilot study of imiquimod 5% cream applied once per week for 6 months for the treatment of actinic keratoses. Journal of the American Academy of Dermatology 2009;60(1):59‐62. [PUBMED: 18937999 ] - PubMed
References to studies excluded from this review
Alberts 2004 {published data only}
-
- Alberts D, Ranger‐Moore J, Einsphahr J, Saboda K, Bozzo P, Liu Y, et al. Safety and efficacy of dose‐intensive oral vitamin A in subjects with sun‐damaged skin. Clinical Cancer Research 2004;10(6):1875‐80. [PUBMED: 15041701] - PubMed
Alexiades‐Armenakas 2003 {published data only}
-
- Alexiades‐Armenakas MR, Geronemus RG. Laser‐mediated photodynamic therapy of actinic keratoses. Archives of Dermatology 2003;139(10):1313‐20. [PUBMED: 14568836] - PubMed
Apalla 2010a {unpublished data only}
-
- Apalla Z, Panagiotidou D, Ioannides D, Sotiriou E. Does the fluence rate influence clinical outcome and pain experienced by the patients with actinic keratosis treated with PDT?. Melanoma Research 2010;20(e‐suppl):e73.
Apalla 2010b {unpublished data only}
-
- Apalla Z, Panagiotidou D, Ioannides D, Sotiriou E. Which light dose is the optimal for treating actinic keratosis with PDT? An intraindividual randomized active‐controlled study. Abstracts of the 6th Congress of the European Association of Dermatologic Oncology [P70]. Melanoma Research 2010;20(e‐Suppl):e73‐4.
Apalla 2010c {published data only}
-
- Apalla Z, Sotiriou E, Chovarda E, Lefaki I, Devliotou‐Panagiotidou D, Ioannides D. Skin cancer: preventive photodynamic therapy in patients with face and scalp cancerization. A randomized placebo‐controlled study. British Journal of Dermatology 2010;162(1):171‐5. [PUBMED: 19863513] - PubMed
Babilas 2006 {published data only}
-
- Babilas P, Kohl E, Maisch T, Backer H, Grob B, Branzan AL, et al. In vitro and in vivo comparison of two different light sources for topical photodynamic therapy. British Journal of Dermatology 2006;154(4):712‐718. [PUBMED: 16536815 ] - PubMed
Babilas 2007 {published data only}
-
- Babilas P, Knobler R, Hummel S, Gottschaller C, Maisch T, Koller M, et al. Variable pulsed light is less painful than light‐emitting diodes for topical photodynamic therapy of actinic keratosis: a prospective randomized controlled trial. British Journal of Dermatology 2007;157(1):111‐7. [PUBMED: 17542980 ] - PubMed
Babilas 2008 {published data only}
-
- Babilas P, Travnik R, Werner A, Landthaler M, Szeimies RM. Split‐face‐study using two different light sources for topical PDT of actinic keratoses:non‐inferiority of the LED system. Journal der Deutschen Dermatologischen Gesellschaft 2008;6(1):25‐32. [PUBMED: 17995967 ] - PubMed
Bartels 2009 {published data only}
-
- Bartels P, Yozwiak M, Einspahr J, Saboda K, Liu Y, Brooks C, et al. Chemopreventive efficacy of topical difluoromethylornithine and/or triamcinolone in the treatment of actinic keratoses analyzed by karyometry. Analytical & Quantitative Cytology & Histology 2009;31(6):355‐66. [PUBMED: 20698351 ] - PMC - PubMed
Berlin 2008 {published data only}
-
- Berlin JM, Rigel DS. Diclofenac sodium 3% gel in the treatment of actinic keratoses postcryosurgery. Journal of Drugs in Dermatology 2008;7(7):669‐73. [PUBMED: 18664159] - PubMed
Biecha‐Thalharnmer 2003 {unpublished data only}
-
- Biecha‐Thalharnmer U, Honigsmann H, Tanew A. Comparison of incoherent versus pulsed monochromatic light for photodynamic therapy of actinic keratoses. [Abstract FC13‐3]. The 12th Congress of the European Academy of Dermatology and Venereology. Barcelona, Spain 15‐18th October 2003. Journal of the European Academy of Dermatology & Venereology 2003;17(Suppl 3):143.
Braathen 2009 {published data only}
-
- Braathen LR, Paredes BE, Saksela O, Gardio K, Morken T, Florich KW, et al. Short incubation with methyl aminolevulinate for photodynamic therapy of actinic keratoses. European Academy of Dermatology & Venereology 2009;23(5):550‐555. [PUBMED: 19415804] - PubMed
Breza 1976 {published data only}
-
- Breza T, Taylor JR, Eaglestein WH. Noninflammatory destruction of actinic keratoses by fluorouracil. Archives of Dermatology 1976;112(9):1256‐8. [PUBMED: 999302] - PubMed
Dermik 2003 {published data only}
-
- Dermik. Product monograph: Carac Cream 0.5% (fluorouracil cream). carac.info/hcp/efficacy.aspx (accessed 26 April 2011).
de Sévaux 2003 {published data only}
-
- Sévaux R, Smit J, Jong E, Kerkhof P, Hoitsma A. Acitretin treatment of premalignant and malignant skin disorders in renal transplant recipients: Clinical effects of a randomized trial comparing two doses of acitretin. Journal of the American Academy of Dermatology 2003;49:407‐12. [PUBMED: 12963902] - PubMed
Dirschka 2010 {published and unpublished data}
-
- Dirschka T, Bierhoff E, Pflugfelder A, Garbe C. 3.0% diclofenac in 2.5% hyaluronic acid inverts the process of cancerous transformation in actinic keratoses and maintains therapeutic response by prolongation of treatment. Melanoma Research 2010;20(e‐suppl):e39.
-
- Dirschka T, Bierhoff E, Pflugfelder A, Garbe C. Topical 3.0% diclofenac in 2.5% hyaluronic acid gel induces regression of cancerous transformation in actinic keratoses. Journal of the European Academy of Dermatology & Venereology 2010;24(3):258‐63. [PUBMED: 19709346] - PubMed
DUSA 2009 {unpublished data only}
-
- NCT00865878. ALA‐PDT Versus Vehicle PDT for Treatment of AK and Reduction of New NMSC in Solid Organ Transplant Recipients. clinicaltrials.gov/ct2/show/NCT00865878 (accessed 14 March 2011).
Edwards 1986 {published data only}
-
- Edwards L, Levine N, Weidner M, Piepkorn M, Smiles K. Effect of intralesional interferon on actinic keratoses. Archives of Dermatology 1986;122(7):779‐82. [PUBMED: 3524471 ] - PubMed
Elmets 2010 {published data only}
Epstein 2006 {published data only}
-
- Epstein E. Twice daily vs. four times daily 5‐fluorouracil therapy for actinic keratoses: a split face study. British Journal of Dermatology 2006;154(4):794‐5. [PUBMED: 16536841] - PubMed
Ericson 2004 {published data only}
-
- Ericson M, Sandburg C, Stenquist B, Gudmundson F, Karlsson M, Ros AM, et al. Photodynamic therapy of actinic keratosis at varying fluence rates: assessment of photobleaching, pain and primary clinical outcome. British Journal of Dermatology 2004;151(6):1204‐12. [PUBMED: 15606516] - PubMed
Fowler 2002 {published data only}
-
- Fowler JF, Zax RH. Aminolevulinic acid hydrochloride with photodynamic therapy: efficacy outcomes and recurrence 4 years after treatment. Cutis 2002;69(6):2‐7. [PUBMED: 12095067] - PubMed
Gold 2006 {published data only}
-
- Gold MH, Bradshaw VL, Boring MM, Bridges TM, Biron JA. Split‐face comparison of photodynamic therapy with 5‐aminolevulinic acid and intense pulsed light versus intense pulsed light alone for photodamage. Dermatologic Surgery 2006;32(6):795‐801. [PUBMED: 16792644 ] - PubMed
Goldman 2003 {published data only}
-
- Goldman MP, Atkin DH. ALA‐PDT in the treatment of actinic keratosis: spot versus confluent therapy. Journal of Cosmetic & Laser Therapy 2003;5(2):107‐10. [PUBMED: 12850802] - PubMed
Green 1998 {published data only}
-
- Green C, Orchard G, Cerio R, Hawk JL. A clinicopathological study of the effects of topical retinyl propionate cream in skin photoageing. Clinical & Experimental Dermatology 1998;23(4):162‐7. [PUBMED: 9894360] - PubMed
Griffin 1991 {published data only}
-
- Griffin TD, Scott EJ. Use of pyruvic acid in the treatment of actinic keratoses: a clinical and histopathological study. Cutis 1991;47(5):325‐9. [PUBMED: 2070654] - PubMed
Grimaître 2000 {published data only}
-
- Grimaitre M, Etienne A, Fathi M, Piletta PA, Saurat JH. Topical colchicine therapy for actinic keratoses. Dermatology 2000;200(4):346‐8. [PUBMED: 10894974] - PubMed
Gupta 2004 {unpublished data only}
-
- Gupta AK. Single‐blind, randomized controlled trial to evaluate the efficacy and safety of photodynamic therapy using aminolevulinic acid versus 5‐fluorouracil in the management of actinic keratosis. Journal of the American Academy of Dermatology 2004;50(S3):P129.
Hanke 2011 {published data only}
-
- Hanke CW, Swanson N, Bruce S, Berman B, Kulp J, Levy S. Complete clearance is sustained for at least 12 months after treatment of actinic keratoses of the face or balding scalp via daily dosing with imiquimod 3.75% or2.5% cream. Journal of Drugs in Dermatology 2011;10(2):165‐70. [PUBMED: 21283921] - PubMed
Humphreys 1996 {published data only}
-
- Humphreys TR, Werth V, Dzubow L, Kligman A. Treatment of photodamaged skin with trichloroacetic acid and topical tretinoin. Journal of the American Academy of Dermatology 1996;34(4):638‐44. [PUBMED: 8601654 ] - PubMed
Jury 2005 {published data only}
-
- Jury CS, Ramraka‐Jones VS, Gudi V, Herd RM. A randomized trial of topical 5% 5‐fluorouracil (Efudix cream) in the treatment of actinic keratoses comparing daily with weekly treatment. British Journal of Dermatology 2005;153(4):808‐810. [PUBMED: 16181465 ] - PubMed
Kurwa 1999 {published data only}
-
- Kurwa HA, Yong‐Gee SA, Seed PT, Markey AC, Barlow RJ. A randomized paired comparison of photodynamic therapy and topical 5‐fluorouracil in the treatment of actinic keratoses. Journal of the American Academy of Dermatology 1999;41(3 Pt 1):414‐8. [PUBMED: 10459115] - PubMed
Marrero 1998 {published data only}
-
- Marrero GM, Katz BE. The new fluor‐hydroxy pulse peel: a combination of 5‐fluorouracil and glycolic acid. Dermatological Surgery 1998;24(9):973‐8. [PUBMED: 9754085 ] - PubMed
Morales 2010 {published data only}
-
- Morales Toquero A, Ocampo Candiani J, Gomez Flores M, Gonzalez Gonzalez SE, Eguia Rodriguez R, Mendez Olvera NP, et al. Clinical and histological evaluation of 5% imiquimod cream vs 5% 5‐fluorouracil ointment in patients with actinic keratosis on the face [Evaluación clínica e histológica de imiquimod a 5% en crema vs5‐fluorouracilo a 5% en ungüento en pacientes con queratosisactínicas en la cara]. Dermatologia Revista Mexicana 2010;54(6):326‐31.
Naylor 1995 {published data only}
-
- Naylor MF, Boyd A, Smith DW, Cameron GS, Hubbard D, Neldner KH. High sun protection factor sunscreens in the suppression of actinic neoplasia. Archives of Dermatology 1995;131(2):170‐5. [PUBMED: 7857113] - PubMed
NCT00005097 {unpublished data only}
-
- NCT00005097. Green Tea Extract in Treating Patients With Actinic Keratosis. clinicaltrials.gov/ct2/show/NCT00005097 (accessed 14 March 2011).
Puizina‐Ivic 2008a {published data only}
-
- Puizina‐Ivic N, Zorc H, Vanjaka‐Rogosic L, Miric L, Persin A. Fractionated illumination improves the outcome in the treatment of precancerous lesions with photodynamic therapy. Collegium Antropologicum. 2008;32(Suppl 2):67‐73. [PUBMED: 19138010] - PubMed
Radakovic‐Fijan 2005 {published data only}
-
- Radakovic‐Fijan S, Blecha‐Thalhammer U, Kittler H, Honigsmann H, Tanew A. Efficacy of 3 different light doses in the treatment of actinic keratoses with 5‐aminolevulinic acid photodynamic therapy: a randomized, observer‐blinded, intrapatient, comparison study. Journal of the American Academy of Dermatology 2005;53:823‐7. [PUBMED: 16243131 ] - PubMed
Robins 2002a {published data only}
-
- Robins P. Pulse therapy with 5‐FU in eradication actinic keratoses with less than recommended dosage. Journal of Drugs in Dermatology 2002;1(1):25‐30. [PUBMED: 12847751] - PubMed
Rosen 2010 {unpublished data only}
-
- Rosen R. Clinical development of ingenol mebutate (PEP005) gel for actinic keratosis. 43rd Annual Scientific Meeting of the Australasian College of Dermatologists Darwin, NT Australia. Australasian Journal of Dermatology 2010; Vol. 51, issue Suppl 1:A5.
Shuttleworth 1989 {published data only}
-
- Shuttleworth D, Marks R. A comparison of the effects of intralesional interferon a‐2b and topical 5% 5‐fluorouracil cream in the treatment of solar keratoses and Bowen's disease. Journal of Dermatological Treatment 1989;1(2):65‐8.
Simmonds 1973 {published data only}
-
- Simmonds WL. Double‐blind investigation comparing a 1%‐vs‐5% 5‐fluorouracil topical cream in patients with multiple actinic keratoses. Cutis 1973;12(4):615‐8.
Smith 2006 {published data only}
-
- Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5‐fluorouracil cream in the treatment of actinic keratoses of the face and scalp. Journal of Drugs in Dermatology 2006;5(2):156‐9. [PUBMED: 16485883] - PubMed
Sotiriou 2011 {published data only}
-
- Sotiriou E, Apalla Z, Chovarda E, Goussi C, Trigoni A, Ioannides D. Single vs. fractionated photodynamic therapy for face and scalp actinic keratoses: a randomized, intraindividual comparison trial with 12‐month follow‐up. Journal of the European Academy of Dermatology & Venereology 2011 Mar 2 [Epub ahead of print]. [PUBMED: 21366709] - PubMed
Spencer 2010 {published data only}
-
- Spencer J. Multicenter, randomized, double‐blind, vehicle‐controlled, dose‐ranging study to evaluate the efficacy and safety of PEP005 (ingenol mebutate) gel 0.005%, 0.01%, and 0.015% when used to treat actinic keratoses on the head. Journal of the American Academy of Dermatology 2010; Vol. 62, issue 3 Suppl 1:AB105.
Stockfleth 2004 {published data only}
-
- Stockfleth E, Christophers E, Benninghoff B, Sterry W. Low incidence of new actinic keratoses after topical 5% imiquimod cream treatment: a long‐term follow‐up study. Archives of Dermatology 2004;140(12):1542. [PUBMED: 15611446 ] - PubMed
Szeimies 2010a {published data only}
-
- Szeimies RM, Stockfleth E, Popp G, Borrosch F, Bruning H, Dominicus R, et al. Long‐term follow‐up of photodynamic therapy with a self‐adhesive 5‐aminolaevulinic acid patch: 12 months data. British Journal of Dermatology 2010;162(2):410‐4. [PUBMED: 19804593] - PubMed
Touma 2004 {published data only}
-
- Paquet M. Touma et al. 2004 [personal communication]. Email to: BA Gilchrest 29 March 2011.
-
- Touma D, Yaar M, Whitehead S, Konnikov N, Gilchrest B. A Trial of Short Incubation, Broad‐Area Photodynamic Therapy for Facial Actinic Keratoses and Diffuse Photodamage. Archives of Dermatology 2004;140(1):33‐40. [PUBMED: 14732657] - PubMed
Tsoukas 2010 {unpublished data only}
-
- Tsoukas M. Treatment of actinic keratosis with 5‐ALA PDT versus topical imiquimod in healthy and immunosuppressed patients. Melanoma Research 2010;20(e‐suppl):e39‐40.
Valeant 2004 {published data only}
-
- Valeant. Efudex topical solutions and cream (fluorouracil). Product monograph. valeant.com/fileRepository/products/PI/Efudex‐40_Cream_5_Solution_2‐5_PI... (accessed 26 April 2011).
Vbeam 2005 {unpublished data only}
-
- NCT00524485. Photodynamic Therapy Using Topical Aminolevulinic Acid in Treating Patients With Actinic Keratosis. clinicaltrials.gov/ct2/show/NCT00524485 (accessed 14 March 2011).
Weinstock 2010 {unpublished data only}
-
- Weinstock MA, Bingham SF. High‐dose topical tretinoin for reducing multiplicity of actinic keratoses. 2010 Annual Meeting of the Society for Investigative Dermatology Atlanta, GA United States. Journal of Investigative Dermatology 2010; Vol. Suppl 1, issue 130:S63.
Wennberg 2008 {published data only}
-
- Wennberg AM, Stenquist B, Stockfleth E, Keohane S, Lear JT, Jemec G, et al. Photodynamic therapy with methyl aminolevulinate for prevention of new skin lesions in transplant recipients: a randomized study. Transplantation 2008;86(3):423‐9. [PUBMED: 18698246] - PubMed
Wulf 2006 {published data only}
-
- Wulf HC, Pavel S, Stender I, Bakker‐Wensveen CA. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Dermato‐Venereologica 2006;86(1):25‐8. [PUBMED: 16585985] - PubMed
Yamauchi 2002 {published data only}
-
- Yamauchi PS, Lowe NJ, Lask GP, Patnaik R, Moore D, Foley P. Methyl aminolevulinate and photodynamic therapy in the treatment of actinic keratosis. [Abstract 804]. The 63rd annual meeting of the Society for Investigative Dermatology, 15‐18 May, Los Angeles, USA. Journal of Investigative Dermatology 2002; Vol. 119, issue 1:341.
References to studies awaiting assessment
Akarsu 2011 {published data only}
-
- Akarsu S, Aktan S, Atahan A, Koc P, Ozkan S. Comparison of topical 3% diclofenac sodium gel and 5% imiquimod cream for the treatment of actinic keratoses. Clinical & Experimental Dermatology 2011;36(5):479‐84. [PUBMED: 21418281] - PubMed
Apalla 2011 {published data only}
-
- Apalla Z, Sotiriou E, Panagiotidou D, Lefaki I, Goussi C, Ioannides D. The impact of different fluence rates on pain and clinical outcome in patients with actinic keratoses treated with photodynamic therapy. Photodermatology, Photoimmunology & Photomedicine 2011;27(4):181‐5. [PUBMED: 21729165] - PubMed
Azimi 2012 {published data only}
-
- Azimi H, Zadeh MG, Jaberian M, Omid M. Comparison of the efficacy of cryotherapy and 0.1% Acnalen gel vs. cryotherapy and placebo in the treatment of actinic keratoses. Pakistan Journal of Medical Sciences 2012;28(1):54‐7.
-
- IRCT201010104901N1. Comparison of the efficacy of cryotherapy and 0.1 % acnalen gel vs. cryotherapy alone in the treatment of actinic keratoses: a randomized, double‐blind clinical trial. irct.ir/searchresult.php?id=4901&number=1 (accessed 14 March 2011).
Damian 2011 {unpublished data only}
-
- ACTRN12609000490279. Randomized, double‐blind, placebo controlled study to assess efficacy of oral nicotinamide in the treatment and prevention of actinic keratoses. anzctr.org.au/trial_view.aspx?ID=83879 (accessed 14 March 2011).
-
- Damian D, Surjana D, Martin A, Halliday G. Oral nicotinamide for skin cancer prevention. 41st Annual Meeting of the European Society for Dermatological Research, ESDR 2011 Barcelona Spain. 7‐10 September 2011. Journal of Investigative Dermatology 2011;131(Suppl 2):S99. - PubMed
Deonizio 2011 {published data only}
-
- Deonizio JM, Mulinari‐Brenner FA. Cryopeeling for treatment of photodamage and actinic keratosis: liquid nitrogen versus portable system. Anais Brasileiros De Dermatologia 2011;86(3):440‐4. [PUBMED: 21738958] - PubMed
Dirschka 2012 {published data only}
-
- Dirschka T, Radny P, Dominicus R, Mensing H, Bruning H, Jenne L, et al. Photodynamic therapy with BF‐200 ALA for the treatment of actinic keratosis: Results of a multicentre, randomized, observer‐blind phase III study in comparison with a registered methyl‐5‐aminolaevulinate cream and placebo. British Journal of Dermatology 2012;166(1):137‐46. [PUBMED: 21910711] - PubMed
Galitzer 2011 {published data only}
-
- Galitzer BI. Effect of retinoid pretreatment on outcomes of patients treated by photodynamic therapy for actinic keratosis of the hand and forearm. Journal of Drugs in Dermatology 2011;10(10):1124‐32. [PUBMED: 21968662] - PubMed
Haddad 2011 {published data only}
-
- Haddad A, Santos ID, Gragnani A, Ferreira LM. The effect of increasing fluence on the treatment of actinic keratosis and photodamage by photodynamic therapy with 5‐aminolevulinic acid and intense pulsed light. Photomedicine & Laser Surgery 2011;29(6):427‐32. [PUBMED: 21631378] - PubMed
Lebwohl 2012 {unpublished data only}
-
- Anderson L, Melgaard A, Schmeider G, Xu Z. Two‐day topical treatment with ingenol mebutate gel, 0.05% for actinic keratoses on the trunk and extremities: Analysis of data pooled from two trials. 70th Annual Meeting of the American Academy of Dermatology San Diego, CA United States, 16‐20 March 2012. Journal of the American Academy of Dermatology 2012;66(4 Suppl):AB159.
-
- Berman B, Melgaard A, Marmur E, Larsson T. Three‐day topical treatment with ingenol mebutate gel, 0.015% for actinic keratoses on the face and scalp: analysis of data pooled from two trials. 70th Annual Meeting of the American Academy of Dermatology San Diego, CA United States, 16‐20 March 2012. Journal of the American Academy of Dermatology 2012;66(4 Suppl):AB3.
-
- Lebwohl M, Melgaard A, Kobayashi K, Swanson N. Local skin responses associated with ingenol mebutate gel for the treatment of actinic keratosis: Two analyses of pooled data, 70th Annual Meeting of the American Academy of Dermatology San Diego, CA United States, 16‐20 March 2012. Journal of the American Academy of Dermatology 2012;66(4 Suppl):AB153.
-
- Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman B. Ingenol mebutate gel for actinic keratosis. New England Journal of Medicine 2012;366(11):1010‐9. [PUBMED: 22417254] - PubMed
-
- Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman B. Ingenol mebutate gel for actinic keratosis‐online supplement. New England Journal of Medicine 2012; Vol. 366, issue 11. [PUBMED: 22417254] - PubMed
Serra‐Guillen 2012 {published data only}
-
- Serra‐Guillen C, Nagore E, Hueso L, Traves V, Messeguer F, Sanmartin O, et al. A randomized pilot comparative study of topical methyl aminolevulinate photodynamic therapy versus imiquimod 5% versus sequential application of both therapies in immunocompetent patients with actinic keratosis: Clinical and histologic outcomes. Journal of the American Academy of Dermatology 2012;66(4):e131‐7. [PUBMED: 22226430] - PubMed
Stockfleth 2011 {published data only}
-
- NCT00987246. Study on the efficacy of LAS41005 in the treatment of actinic keratosis. clinicaltrials.gov/ct2/show/NCT00987246ls.gov (accessed 14 March 2011).
-
- Stockfleth E, Kerl H, Zwingers T, Willers C. Low‐dose 5‐fluorouracil in combination with salicylic acid as a new lesion‐directed option to treat topically actinic keratoses: histological and clinical study results. British Journal of Dermatology 2011;165(5):1101‐8. [PUBMED: 21517801] - PubMed
Wiegell 2011b {published data only}
-
- Wiegell SR, Heydenreich J, Fabricius S, Wulf HC. Continuous ultra‐low‐intensity artificial daylight is not as effective as red LED light in photodynamic therapy of multiple actinic keratoses. Photodermatology, Photoimmunology & Photomedicine 2011;27(6):280‐5. [PUBMED: 22092730] - PubMed
-
- Willey A. Temperature‐modulated photodynamic therapy for the treatment of actinic keratoses on the extremities. 70th Annual Meeting of the American Academy of Dermatology San Diego, CA United States, 16‐20 March 2012. Journal of the American Academy of Dermatology 2012;66(4 Suppl):AB177.
References to ongoing studies
ACTRN12610000689077 {unpublished data only}
-
- ACTRN12610000689077. Effect of nicotinamide versus placebo on numbers of actinic keratoses. anzctr.org.au/trial_view.aspx?ID=335868 (accessed 14 March 2011).
NCT00115154 {unpublished data only}
-
- NCT00115154. Study to assess the safety and efficacy of imiquimod 5% cream for the treatment of actinic keratosis on the arms and hands. clinicaltrials.gov/ct2/show/NCT00115154 (accessed 14 March 2011).
NCT00204542 {unpublished data only}
-
- NCT00204542. Comparison of the efficacy and tolerability of Solaraze for 3 versus 6 months in patients with mild to moderate actinic keratosis located on the face and head. clinicaltrials.gov/ct2/show/NCT00204542 (accessed 14 March 2011).
NCT00472459 {unpublished data only}
-
- NCT00472459. PDT with Metvix® 160 mg/g cream in organ transplant recipients with non‐melanoma skin cancer. clinicaltrials.gov/ct2/show/NCT00472459 (accessed 14 March 2011).
NCT00608634 {unpublished data only}
-
- NCT00608634. Topical perillyl alcohol in treating patients with sun damaged skin and actinic keratoses. clinicaltrials.gov/ct2/show/NCT00608634 (accessed 14 March 2011).
NCT00695578 {unpublished data only}
-
- NCT00695578. Clinical trial to evaluate biafine cream versus standard care in subjects with actinic keratosis post cryotherapy. clinicaltrials.gov/ct2/show/NCT00695578 (accessed 14 March 2011).
NCT00700063 {unpublished data only}
-
- NCT00700063. A multicenter study to evaluate the safety and efficacy of PEP005 topical gel when used to treat actinic keratoses on the head (face or scalp). clinicaltrials.gov/ct2/show/NCT00700063 (accessed 14 March 2011).
NCT00756288 {unpublished data only}
-
- NCT00756288. Photo‐therapy with a topical retinoid versus photo‐therapy alone for actinic keratoses. clinicaltrials.gov/ct2/show/NCT00756288 (accessed 14 March 2011).
NCT00786994 {unpublished data only}
-
- NCT00786994. The efficacy and tolerability of oleogel‐S‐10 in patients with actinic keratoses. clinicaltrials.gov/ct2/show/NCT00786994 (accessed 14 March 2011).
NCT00859105 {unpublished data only}
-
- NCT00859105. A randomized, double‐blind, parallel‐group, vehicle‐controlled therapeutic equivalence study of three Imiquimod cream 5% treatments for patients with actinic keratosis. clinicaltrials.gov/ct2/show/NCT00859105 (accessed 14 March 2011).
NCT00948428 {unpublished data only}
-
- NCT00948428. Bioequivalence of generic imiquimod cream, 5% when compared to Aldara™ (imiquimod) cream, 5% in the treatment of actinic keratosis. clinicaltrials.gov/ct2/show/NCT00948428 (accessed 14 March 2011).
NCT00991861 {unpublished data only}
-
- NCT00991861. Efficacy and safety study of LAS41007 in the treatment of actinic keratosis. clinicaltrials.gov/ct2/show/NCT00991861 (accessed 14 March 2011).
NCT01203878 {unpublished data only}
-
- NCT01203878. Treatment of actinic keratoses of the face with imiquimod 3.75% cream followed by photodynamic therapy. clinicaltrials.gov/ct2/show/NCT01203878 (accessed 14 March 2011).
NCT01229319 {unpublished data only}
-
- NCT01229319. Imiquimod 3.75% cream in combination with cryotherapy in the treatment of hypertrophic actinic keratoses. clinicaltrials.gov/ct2/show/NCT01229319 (accessed 14 March 2011).
NCT01260987 {unpublished data only}
-
- NCT01260987. Fractional CO2 laser assisted photodynamic therapy. clinicaltrials.gov/ct2/show/NCT01260987 (accessed 14 March 2011).
NCT01265602 {unpublished data only}
-
- NCT01265602. Double‐blind, randomized, vehicle‐ and comparator‐controlled, multi‐center trial to evaluate the efficacy and safety of LAS41007 in the treatment of actinic keratosis. clinicaltrials.gov/ct2/show/NCT01265602 (accessed 14 March 2011).
NCT01354717 {unpublished data only}
-
- NCT01354717. Bioequivalence study of generic fluorouracil 0.5% cream and 0.5% Carac® and placebo. clinicaltrials.gov/ct2/results?term=NCT01354717 (accessed 5 April 2012).
NCT01358851 {unpublished data only}
-
- NCT01358851. LAS41005 in hyperkeratotic actinic keratosis. clinicaltrials.gov/ct2/results?term=NCT01358851 (accessed 5 April 2012).
NCT01413763 {unpublished data only}
-
- NCT01413763. Potential effect of topical imiquimod on atrial ectopy in patients with actinic aeratosis. clinicaltrials.gov/ct2/results?term=NCT01413763 (accessed 5 April 2012).
NCT01453179 {unpublished data only}
-
- NCT01453179. Long‐term effects of imiquimod and diclofenac in actinic keratoses (LEIDA 2). clinicaltrials.gov/ct2/results?term=NCT01453179 (accessed 5 April 2012).
NCT01458587 {unpublished data only}
-
- NCT01458587. Levulan PDT versus vehicle for extremity actinic keratoses (AK). clinicaltrials.gov/ct2/results?term=NCT01458587 (accessed 5 April 2012).
NCT01459393 {unpublished data only}
-
- NCT01459393. Comparison between 5‐aminolevulinic acid photodynamic therapy versus cryotherapy for actinic keratosis treatment. clinicaltrials.gov/ct2/results?term=NCT01459393 (accessed 5 April 2012).
NCT01475071 {unpublished data only}
-
- NCT01475071. Intra‐individual comparison of efficacy and safety of Metvix® natural daylight photodynamic therapy versus conventional Metvix® photodynamic therapy in subject with mild actinic keratoses (CoMet). clinicaltrials.gov/ct2/results?term=NCT01475071 (accessed 5 April 2012).
NCT01475955 {unpublished data only}
-
- NCT01475955. Short‐incubation Levulan photodynamic therapy versus vehicle for face/scalp actinic keratosis (AK). clinicaltrials.gov/ct2/results?term=NCT01475955 (accessed 5 April 2012).
NCT01481155 {unpublished data only}
-
- NCT01481155. Comparative study of photodynamic therapy vs. CO2 laser therapy in treatment of actinic keratoses. clinicaltrials.gov/ct2/results?term=NCT01481155 (accessed 5 April 2012).
NCT01493921 {unpublished data only}
-
- NCT01493921. Efficacy study & safety evaluation of SR‐T100 gel in actinic keratosis treatment. clinicaltrials.gov/ct2/results?term=NCT01493921 (accessed 5 April 2012).
NCT01502020 {unpublished data only}
-
- NCT01502020. A bioequivalence study with clinical endpoints comparing generic imiquimod cream, 3.75% and Zyclara™ (imiquimod) cream, 3.75% in subjects with actinic keratoses. clinicaltrials.gov/ct2/results?term=NCT01502020 (accessed 5 April 2012).
NCT01516515 {unpublished data only}
-
- NCT01516515. Efficacy and safety phase II dose‐ranging study of SR‐T100 to treat actinic keratosis. clinicaltrials.gov/ct2/results?term=NCT01516515 (accessed 5 April 2012).
NCT01525329 {unpublished data only}
-
- NCT01525329. Combination therapy with 5‐FU and PDT for the treatment of post‐transplant premalignant skin disease. clinicaltrials.gov/ct2/results?term=NCT01525329 (accessed 5 April 2012).
NCT01538901 {unpublished data only}
-
- NCT01538901. Imiquimod versus photodynamic therapy of actinic keratoses in organ transplant recipients. clinicaltrials.gov/ct2/results?term=NCT01538901 (accessed 5 April 2012).
NCT01541228 {unpublished data only}
-
- NCT01541228. Clinical effect of photodynamic treatment when treating actinic keratoses with different light doses. clinicaltrials.gov/ct2/results?term=NCT01541228 (accessed 5 April 2012).
NCT01541553 {unpublished data only}
-
- NCT01541553. A sequential treatment regimen of cryotherapy and Picato® for the treatment of actinic keratosis on the face and scalp. clinicaltrials.gov/ct2/results?term=NCT01541553 (accessed 5 April 2012).
Willey 2011 {unpublished data only}
-
- Willey A, Sakamoto F. Temperature modulated photodynamic therapy for the treatment of actinic keratoses on the extremities. 31st Annual Conference of the American Society for Laser Medicine and Surgery, ASLMS 2011 Grapevine, TX United States, 30/3‐3/4 2011. Lasers in Surgery & Medicine 2011;43(S23):957‐958.
Additional references
Adamson 2002
-
- Adamson DJ, Frew D, Tatoud R, Wolf CR, Palmer CN. Diclofenac antagonizes peroxisome proliferator‐activated receptor‐gamma signaling. Molecular Pharmacology 2002;61(1):7‐12. - PubMed
Alam 1995
-
- Alam CA, Seed MP, Willoughby DA. Angiostasis and vascular regression in chronic granulomatous inflammation induced by diclofenac in combination with hyaluronan in mice. Journal of Pharmacy & Phamacology 1995;47(5):407‐11. - PubMed
Amini 2010
Askew 2009
-
- Askew DA, Mickan SM, Soyer HP, Wilkinson D. Effectiveness of 5‐fluorouracil treatment for actinic keratosis‐‐a systematic review of randomized controlled trials. International Journal of Dermatology 2009;48(5):453‐63. [PUBMED: 19416373] - PubMed
Berman 2006
-
- Berman B, Villa AM, Ramirez CC. Mechanisms of action of new treatment modalities for actinic keratosis. Journal of Drugs in Dermatology 2006;5(2):167‐73. [PUBMED: 16485885] - PubMed
Brown 2005
-
- Brown MB, Jones SA. Hyaluronic acid: a unique topical vehicle for the localized delivery of drugs to the skin. Journal of the European Academy of Dermatology & Venereology 2005;19:308‐18. [PUBMED: 15857456] - PubMed
Callen 1997
-
- Callen JP, Bickers DR, Moy RL. Actinic keratoses. Journal of the American Academy of Dermatology 1997;36(4):650‐3. - PubMed
Chakrabarty 2004
-
- Chakrabarty A, Geisse JK. Medical therapies for non‐melanoma skin cancer. Clinics in Dermatology 2004;22(3):183‐8. - PubMed
Cockerell 2000
-
- Cockerell CJ. Histopathology of incipient intraepidermal squamous cell carcinoma ("actinic keratosis"). Journal of the American Academy of Dermatology 2000;42:S11‐17. [PUBMED: 10607351] - PubMed
Correale 2002
-
- Correale CE. Actinic keratoses: new treatment options. Advances in Dermatology 2002;18:339‐55. - PubMed
Dinehart 2000
-
- Dinehart SM. The treatment of actinic keratoses. Journal of the American Academy of Dermatology 2000;42(1 Pt 2):25‐8. - PubMed
Eaglstein 1970
-
- Eaglstein WH, Weinstein GD, Frost P. Fluorouracil: mechanism of action in human skin and actinic keratoses. Effect on DNA synthesis in vivo. Archives of Dermatology 1970;101(2):132‐9. [PUBMED: 5413250] - PubMed
Engel 1988
-
- Engel A, Johnson M, Haynes SG. Health effects of sunlight exposure in the United States. Results from the first National Health and Nutrition Examination Survey, 1971‐1974. Archives of Dermatology 1988;124(1):72‐9. [PUBMED: 3257372] - PubMed
Fayter 2010
-
- Fayter D, Corbett M, Heirs M, Fox D, Eastwood A. A systematic review of photodynamic therapy in the treatment of pre‐cancerous skin conditions, Barrett's oesophagus and cancers of the biliary tract, brain, head and neck, lung, oesophagus and skin. Health Technology Assessment 2010;14(37):1‐288. [PUBMED: 20663420] - PubMed
Feldman 2011
-
- Feldman SR, Fleischer AB. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis 2011;87:201‐7. [PUBMED: 21644496] - PubMed
Fink‐Puches 1997
-
- Fink‐Puches R, Hofer A, Smolle J, Kerl H, Wolf P. Primary clinical responses and long‐term follow‐up of solar keratoses treated with topically applied 5‐ALA and irradiation by different wavebands of light. Journal of Photochemistry & Photobiology 1997;41(1‐2):145‐51. - PubMed
Frost 2000
-
- Frost C, Williams G, Green A. High incidence and regression rates of solar keratoses in a Queensland community. Journal of Investigative Dermatology 2000;115(2):273‐7. - PubMed
Fulton 1999
-
- Fulton JE, Rahimi AD, Helton P, Dahlberg K, Kelly AG. Disappointing results following resurfacing of facial skin with CO2 lasers for prophylaxis of keratoses and cancers. Dermatologic Surgery 1999;25(9):729‐732. [PUBMED: 10491067] - PubMed
Glogau 2000
-
- Glogau RG. The risk of progression to invasive disease. Journal of the American Academy of Dermatology 2000;42(1 Pt 2):23‐4. - PubMed
Gold 2008
-
- Gold MH. Continuing medical education article‐skin treatment: Photodynamic therapy: indications and treatment. Aesthetic Surgery Journal 2008;28(5):545‐52. - PubMed
Goldberg 2010
-
- Goldberg LH, Kaplan B, Vergilis‐Kalner I, Landau J. Liquid nitrogen: temperature control in the treatment of actinic keratosis. Dermatologic Surgery 2010;36(12):1956‐61. - PubMed
Hadley 2006
-
- Hadley G, Derry S, Moore RA. Imiquimod for actinic keratosis: systematic review and meta‐analysis. Journal of Investigative Dermatology 2006;126(6):1251‐55. - PubMed
Hemmi 2002
-
- Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti‐viral compounds activate immune cells via the TLR7 MyD88‐dependent signaling pathway. Nature Immunology 2002;3(2):196‐200. [PUBMED: 11812998] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Holmes 2007
-
- Holmes C, Foley P, Freeman M, Chong AH. Solar keratosis: epidemiology, pathogenesis, presentation and treatment. Australasian Journal of Dermatology 2007;48(2):67‐76. [PUBMED: 17535191] - PubMed
Ibrahim 2009
Jeffes 2000
-
- Jeffes EW, Tang EH. Actinic keratoses. Current treatment options. American Journal of Clinical Dermatology 2000;1(3):167‐79. - PubMed
Juarranz 2008
-
- Juarranz A, Jaen P, Sanz‐Rodriguez F, Cuevas J, Gonzalez S. Photodynamic therapy of cancer. Basic principles and applications. Clinical & Translational Oncology 2008;10(3):148‐54. - PubMed
Juzeniene 2007
-
- Juzeniene A, Peng Q, Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochemical & Photobiological Sciences 2007;6(12):1234‐45. - PubMed
Kaur 2010
-
- Kaur RR, Alikhan A, Maibach HI. Comparison of topical 5‐fluorouracil formulations in actinic keratosis treatment. Journal of Dermatological Treatment 2010;21(5):267‐71. [PUBMED: 19878034] - PubMed
Lebwohl 2003
-
- Lebwohl M. Actinic keratosis: epidemiology and progression to squamous cell carcinoma. British Journal of Dermatology 2003;149(Suppl 66):31‐33. [PUBMED: 14616345] - PubMed
Lee 2005
-
- Lee PK, Harwell WB, Loven KH, Phillips TJ, Whiting DA, Andres KL, et al. Long‐term clinical outcomes following treatment of actinic keratosis with imiquimod 5% cream. Dermatologic Surgery 2005;31(6):659‐63. [PUBMED: 15996416] - PubMed
Lu 1995
Marks 1993
-
- Marks VJ. Actinic keratosis: a premalignant skin lesion. Otolaryngologic Clinics of North America 1993;26(1):23‐35. [PUBMED: 8433840] - PubMed
Martin 2011
-
- Martin GM. Impact of interval and combination therapies on the management of actinic keratosis: review and clinical considerations. Journal of Dermatological Treatment 2011;22:288‐97. [PUBMED: 20528483] - PubMed
Moan 1991
-
- Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochemistry & Photobiology 1991;53(4):549‐53. - PubMed
Moy 2000
-
- Moy RL. Clinical presentation of actinic keratoses and squamous cell carcinoma. Journal of the American Academy of Dermatology 2000;42(1 Pt 2):8‐10. - PubMed
Nelson 1994
-
- Nelson MA, Einspahr JG, Alberts DS, Balfour CA, Wymer JA, Welch KL, et al. Analysis of the p53 gene in human precancerous actinic keratosis lesions and squamous cell cancers. Cancer Letters 1994;85(1):23‐9. - PubMed
NHANES I
-
- Center for Disease Control and Prevention/ National Center for Health Statistics. National Health and Nutrition Examination Survay (NHANES I). http://www.cdc.gov/nchs/nhanes/nhanesi.htm (accessed 24 August 2010).
Ramsay 2003
-
- Ramsay HM, Fryer AA, Hawley CM, Smith AG, Nicol DL, Harden PN. Factors associated with nonmelanoma skin cancer following renal transplantation in Quenssland Australia. Journal of the American Academy of Dermatology 2003;49(3):397‐406. [PUBMED: 12963901] - PubMed
Rigel 2008
-
- Rigel DS, Cockerell CJ, Carucci J, Wharton J. Actinic keratosis, basal cell carcinoma and squamous cell carcinoma. In: Bolognia JL, Jorizzo JL, Rapini RP editor(s). Dermatology. 2nd Edition. Vol. 2, Mosby Elsevier, 2008:1641‐59. [ISBN 9781416029991]
Rivers 2004
-
- Rivers JK. Topical 3% diclofenac in 2.5% hyaluronan gel for the treatment of actinic keratoses. Skin Therapy Letters 2004;9(1):1‐3. - PubMed
Robins 2002b
-
- Robins P, Gupta AK. The use of topical fluorouracil to treat actinic keratosis. Cutis 2002;70(2 Suppl):4‐7. [PUBMED: 12353679] - PubMed
Roewert‐Huber 2007
-
- Roewert‐Huber J, Stockfleth E, Kerl H. Pathology and pathobiology of actinic (solar) keratosis ‐ an update. British Journal of Dermatology 2007;157(Suppl 2):18‐20. [PUBMED: 18067626] - PubMed
Salasche 2000
-
- Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. Journal of the American Academy of Dermatology 2000;42(1 Pt 2):4‐7. - PubMed
Schwartz 1997
-
- Schwartz RA. The actinic keratosis: a perspective and update. Dermatologic Surgery 1997;23(11):1009‐19. [PUBMED: 9391557] - PubMed
Seed 1997
-
- Seed MP, Brown JR, Freemantle CN, Papworth JL, Colville‐Nash PR, Willis D, et al. The inhibition of colon‐26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Research 1997;57(9):1625‐29. - PubMed
Stanley 1999
-
- Stanley MA. Mechanism of action of imiquimod. Papillomavirus Rep 1999;10(2):23‐9.
Thai 2004
-
- Thai KE, Fergin P, Freeman M, Vinciullo C, Francis D, Spelman L, et al . A prospective study of the use of cryosurgery for the treatment of actinic keratoses. International Journal of Dermatology 2004;43(9):687‐92. - PubMed
Vatve 2007
-
- Vatve M, Ortonne J‐P, Birch‐Machin MA, Gupta G. Management of field change in actinic keratosis. British Journal of Dermatology 2007;157(Suppl 2):21‐24. [PUBMED: 18067627] - PubMed
Vidal 2006
-
- Vidal D. Topical imiquimod: mechanism of action and clinical applications. Mini‐Reviews in Medicinal Chemistry 2006;6(5):499‐503. - PubMed
Warino 2006
-
- Warino L, Tusa M, Camacho F, Teuschler H, Fleischer AB Jr, Feldman SR. Frequency and cost of actinic keratosis treatment. Dermatologic Surgery 2006;32(8):1045‐49. [PUBMED: 16918567] - PubMed
Wilkerson 1984
-
- Wilkerson MG, Goldberg LH, Rapini RP. Actinic keratoses. American Family Physician 1984;30(1):103‐8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

