Creatine for amyotrophic lateral sclerosis/motor neuron disease
- PMID: 23235621
- PMCID: PMC11403570
- DOI: 10.1002/14651858.CD005225.pub3
Creatine for amyotrophic lateral sclerosis/motor neuron disease
Abstract
Background: Creatine, a naturally-occurring nitrogenous organic acid involved in adenosine triphosphate (ATP) production, has been shown to increase survival in mouse models of amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND). Results from human trials, however, have been mixed. Given conflicting results regarding the efficacy of creatine, we conducted a systematic review, which was updated in 2012.
Objectives: To systematically examine the efficacy of creatine efficacy in prolonging ALS survival and in slowing ALS disease progression.
Search methods: We searched the Cochrane Neuromuscular Disease Group Specialized Register (16 July 2012), CENTRAL (2012, issue 7 in the Cochrane Library), MEDLINE (January 1966 to July 2012) and EMBASE (January 1980 to July 2012) for any trial involving creatine in the treatment of ALS. We also contacted experts in the field for any additional studies.
Selection criteria: Randomized trials of treatment with creatine or placebo in patients diagnosed with ALS. Our primary outcome was tracheostomy-free survival time; secondary outcomes were ALS progression as measured by changes in ALS functional rating revised scores (ALSFRS-R) and per cent predicted forced vital capacity (FVC) over time.
Data collection and analysis: Two authors independently selected studies, assessed risk of bias and extracted data. We obtained and analyzed individual participant data from each study.
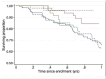
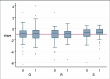
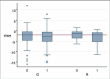
Main results: We included three trials involving 386 participants randomized to either creatine 5 to 10 g per day or placebo. When we updated the searches in 2012 we found no additional trials. Creatine was reportedly well-tolerated in all three included studies, with no evidence of renal failure or serious adverse events specifically attributable to creatine. Using a pooled log-rank statistical test, we found no statistical difference in survival between the placebo and creatine groups across all three studies (Chi(2) = 0.09, P = 0.76). In addition, we found no statistical difference in ALSFRS-R slopes between the two groups across all three studies using a pooled linear mixed-effects model (slope difference of +0.03 ALSFRS-R/month in the creatine group; P = 0.76). Interestingly, there was a trend towards slightly worsened FVC slope in the creatine group (slope difference of -0.63 FVC/month in the creatine group) using a pooled linear mixed-effects model across the two studies which included FVC as an outcome, but this difference was not statistically significant (P = 0.054).
Authors' conclusions: In patients already diagnosed with clinically probable or definite ALS, creatine at doses ranging from 5 to 10 g per day did not have a statistically significant effect on survival, ALSFRS-R progression or percent predicted FVC progression.
Conflict of interest statement
Richard S. Bedlack was a site investigator for a multicenter trial of creatine for ALS (Rosenfeld 2008). He has received consultancy fees from Athena Diagnostics, Avanir, Biogen, Sanofi and UCB and remuneration as a medical monitor for Clinipace and ultragenyx. His institution has grants pending from Biogen, Cytokinetics and Neuraltus Pharmaceuticals. He has received payment for lectures from Avanir, Eli Lilly and Pfizer and for the development of educational presentations from Athena Diagnostics. Daniel M. Pastula has no known conflicts of interest. Dan H. Moore is a biostatistical consultant for several pharmaceutical entities: Neuraltus Pharmaceuticals and Knopp Neurosciences.
Figures
Update of
-
Creatine for amyotrophic lateral sclerosis/motor neuron disease.Cochrane Database Syst Rev. 2010 Jun 16;(6):CD005225. doi: 10.1002/14651858.CD005225.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2012 Dec 12;12:CD005225. doi: 10.1002/14651858.CD005225.pub3. PMID: 20556761 Updated.
Similar articles
-
Creatine for amyotrophic lateral sclerosis/motor neuron disease.Cochrane Database Syst Rev. 2010 Jun 16;(6):CD005225. doi: 10.1002/14651858.CD005225.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2012 Dec 12;12:CD005225. doi: 10.1002/14651858.CD005225.pub3. PMID: 20556761 Updated.
-
Gamma aminobutyric acid (GABA) modulators for amyotrophic lateral sclerosis/motor neuron disease.Cochrane Database Syst Rev. 2017 Jan 9;1(1):CD006049. doi: 10.1002/14651858.CD006049.pub2. Cochrane Database Syst Rev. 2017. PMID: 28067943 Free PMC article.
-
Treatment for sialorrhea (excessive saliva) in people with motor neuron disease/amyotrophic lateral sclerosis.Cochrane Database Syst Rev. 2022 May 20;5(5):CD006981. doi: 10.1002/14651858.CD006981.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593746 Free PMC article.
-
Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease.Cochrane Database Syst Rev. 2017 Oct 6;10(10):CD004427. doi: 10.1002/14651858.CD004427.pub4. Cochrane Database Syst Rev. 2017. PMID: 28982219 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
Cited by
-
Complementary and alternative medicine for treating amyotrophic lateral sclerosis: A narrative review.Integr Med Res. 2019 Dec;8(4):234-239. doi: 10.1016/j.imr.2019.08.003. Epub 2019 Aug 29. Integr Med Res. 2019. PMID: 31692669 Free PMC article. Review.
-
Emergent Prophylactic, Reparative and Restorative Brain Interventions for Infants Born Preterm With Cerebral Palsy.Front Physiol. 2019 Jan 28;10:15. doi: 10.3389/fphys.2019.00015. eCollection 2019. Front Physiol. 2019. PMID: 30745876 Free PMC article. Review.
-
ALS Clinical Trials Review: 20 Years of Failure. Are We Any Closer to Registering a New Treatment?Front Aging Neurosci. 2017 Mar 22;9:68. doi: 10.3389/fnagi.2017.00068. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28382000 Free PMC article. Review.
-
Creatine for women in pregnancy for neuroprotection of the fetus.Cochrane Database Syst Rev. 2014 Dec 19;2014(12):CD010846. doi: 10.1002/14651858.CD010846.pub2. Cochrane Database Syst Rev. 2014. PMID: 25523279 Free PMC article.
-
Creatine as a Neuroprotector: an Actor that Can Play Many Parts.Neurotox Res. 2019 Aug;36(2):411-423. doi: 10.1007/s12640-019-00053-7. Epub 2019 May 8. Neurotox Res. 2019. PMID: 31069754 Review.
References
References to studies included in this review
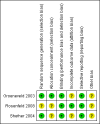
Groeneveld 2003 {published data only}
-
- Groeneveld GJ, Veldink JH, Tweel I, Kalmijn S, Beijer C, Visser M, et al. A randomized sequential trial of creatine in amyotrophic lateral sclerosis. Annals of Neurology 2003;53(4):437‐45. [PUBMED: 12666111] - PubMed
Rosenfeld 2008 {published data only}
Shefner 2004 {published data only}
-
- Shefner JM, Cudkowicz ME, Schoenfeld D, Conrad T, Taft J, Chilton M, et al. NEALS Consortium. A clinical trial of creatine in ALS. Neurology 2004;63(9):1656‐61. [PUBMED: 15534251] - PubMed
References to studies excluded from this review
Rosenfeld 2001 {published data only}
-
- Rosenfeld J. Creatine monohydrate in amyotrophic lateral sclerosis: preliminary results. American Academy of Neurology Annual Meeting. 2001.
Additional references
Adhihetty 2008
Bensimon 1994
-
- Bensimon G, Lacomblez L, Meininger V, ALS/Riluzole Study Group. A controlled trial of riluzole in amyotrophic lateral sclerosis. New England Journal of Medicine 1994;330(9):585‐91. - PubMed
Bessman 1985
-
- Bessman SP, Carpenter CL. The creatine‐creatine phosphate energy shuttle. Annual Review of Biochemistry 1985;54:831‐62. - PubMed
Brooks 2000
-
- Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 2000;1(5):293‐9. - PubMed
Cedarbaum 1999
-
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS‐R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). Journal of the Neurological Sciences 1999;169(1‐2):13‐21. - PubMed
Chancellor 1993
-
- Chancellor AM, Slattery JM, Fraser H, Swingler RJ, Holloway SM, Warlow CP. The prognosis of adult‐onset motor neuron disease: a prospective study based on the Scottish Motor Neuron Disease Register. Journal of Neurology 1993;240(6):339‐46. - PubMed
Cox 1972
-
- Cox D. Regression models and life tables. Journal of the Royal Statistical Society. Series B (Methodological) 1972;34(2):187‐220.
Czaplinski 2006
-
- Czaplinski A, Yen AA, Appel SH. Amyotrophic lateral sclerosis: early predictors of prolonged survival. Journal of Neurology 2006;253(11):1428‐36. - PubMed
Del Aguila 2003
-
- Aguila M, Longstreth WT Jr, McGuire V, Koepsell T, Belle G. Prognosis in amyotrophic lateral sclerosis: a population‐based study. Neurology 2003;60(5):813‐9. - PubMed
Drory 2002
-
- Drory VE, Gross D. No effect of creatine on respiratory distress in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 2002;3(1):43‐6. - PubMed
Goodall 2006
-
- Goodall EF, Morrison KE. Amyotrophic lateral sclerosis (motor neuron disease): proposed mechanisms and pathways to treatment. Expert Reviews in Molecular Medicine 2006;8(11):1‐22. - PubMed
Gordon 2004
-
- Gordon PH, Miller RG, Moore DH. ALSFRS‐R. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 2004;5(Suppl 1):90‐3. - PubMed
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kleopa 1999
-
- Kleopa KA, Sherman M, Neal B, Romano GJ, Heiman‐Patterson T. Bipap improves survival and rate of pulmonary function decline in patients with ALS. Journal of the Neurological Sciences 1999;164(1):82‐8. - PubMed
Klivenyi 1999
-
- Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, et al. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nature Medicine 1999;5(3):347‐50. - PubMed
Lacomblez 1996
-
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Powe L, Durrleman S, et al. A confirmatory dose‐ranging study of riluzole in ALS. ALS/Riluzole Study Group II. Neurology 1996;47(6 (Suppl 4)):S242‐50. - PubMed
Lawler 2002
-
- Lawler JM, Barnes WS, Wu G, Song W, Demaree S. Direct antioxidant properties of creatine. Biochemical & Biophysical Research Communications 2002;290(1):47‐52. - PubMed
Li 1988
Mandrioli 2003
-
- Mandrioli J, Faglioni P, Merelli E, Sola P. The epidemiology of ALS in Modena, Italy. Neurology 2003;60(4):683‐9. - PubMed
Mathus‐Vliegen 1994
-
- Mathus‐Vliegen LMH, Louwerse LS, Merkus MP, Tytgat GNS, Vianney de Jong JMP. Percutaneous endoscopic gastrostomy in patients with amyotrophic lateral sclerosis and impaired pulmonary function. Gastrointestinal Endoscopy 1994;40(4):463‐9. - PubMed
Mazzini 1995
-
- Mazzini L, Corrà T, Zaccala M, Mora G, Piano M, Galante M. Percutaneous endoscopic gastrostomy and enteral nutrition in amyotrophic lateral sclerosis. Neurology 1995;242(10):695‐8. - PubMed
Mazzini 2001
-
- Mazzini L, Balzarini C, Colombo R, Moro G, Pastore I, Ambrogio R, et al. Effects of creatine supplementation on exercise performance and muscular strength in amyotrophic lateral sclerosis: preliminary results. Journal of the Neurological Sciences 2001;191(1‐2):139‐44. - PubMed
Meyer 1984
-
- Meyer RA, Sweeney HL, Kushmerick MJ. A simple analysis of the "phosphocreatine shuttle". American Journal of Physiology 1984;246(5 Pt 1):C365‐77. - PubMed
Miller 2009
-
- Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice parameter update: The care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009;73(15):1218‐26. - PMC - PubMed
Millul 2005
-
- Millul A, Beghi E, Logroscino G, Micheli A, Vitelli E, Zardi A. Survival of patients with amyotrophic lateral sclerosis in a population‐based registry. Neuroepidemiology 2005;25(3):114‐9. - PubMed
Pastula 2009
-
- Pastula DM, Coffman CJ, Allen KD, Oddone EZ, Kasarskis EJ, Lindquist JH, et al. Factors associated with survival in the National Registry of Veterans with ALS. Amyotrophic Lateral Sclerosis 2009;10(5‐6):332‐8. - PubMed
Przedborski 2003
-
- Przedborski S, Mitsumoto H, Rowland LP. Recent advances in amyotrophic lateral sclerosis research. Current Neurology and Neuroscience Reports 2003;3(1):70‐7. - PubMed
Rosen 1993
-
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993;362(6415):59‐62. - PubMed
Rowland 2001
-
- Rowland LP, Schneider NA. Amyotrophic lateral sclerosis. New England Journal of Medicine 2001;344(22):1688‐700. - PubMed
Sanofi‐Aventis 2006
-
- Sanofi‐Aventis. Rilutek prescribing information. Sanofi‐Aventis U.S.LLC, Bridgewater, NJ November 2006.
Sestili 2006
-
- Sestili P, Martinelli C, Bravi G, Piccoli G, Battistelli M, Falcieri E, et al. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radical Biology and Medicine 2006;40(5):837‐49. - PubMed
Siddique 1995
-
- Siddique T, Hentati A. Familial amyotrophic lateral sclerosis. Clinical Neuroscience 1995‐1996;3(6):338‐47. - PubMed
Sorenson 2002
-
- Sorenson EJ, Stalker AP, Kurland LT, Winderbank AJ. Amyotrophic lateral sclerosis in Olmsted County, Minnesota, 1925 to 1998. Neurology 2002;59(2):280‐2. - PubMed
Testa 2004
-
- Testa D, Lovati R, Ferrarini M, Salmoiraghi F, Filippini G. Survival of 793 patients with amyotrophic lateral sclerosis diagnosed over a 28‐year period. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 2004;5(4):208‐12. - PubMed
Traynor 2003
Traynor 2004
-
- Traynor BJ, Zhang H, Shefner JM, Schoenfeld D, Cudkowicz ME, NEALS Consortium. Functional outcome measures as clinical trial endpoints in ALS. Neurology 2004;63(10):1933‐5. - PubMed
Worms 2001
-
- Worms PM. The epidemiology of motor neuron diseases: a review of recent studies. Journal of the Neurological Sciences 2001;191(1‐2):3‐9. - PubMed
References to other published versions of this review
Bedlack 2005
-
- Bedlack RS, Mathew MC. Creatine for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005225] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

