Oral versus intravenous steroids for treatment of relapses in multiple sclerosis
- PMID: 23235634
- PMCID: PMC12145957
- DOI: 10.1002/14651858.CD006921.pub3
Oral versus intravenous steroids for treatment of relapses in multiple sclerosis
Abstract
Background: This is an updated Cochrane review of the previous version published (Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No.: CD006921. DOI: 10.1002/14651858.CD006921.pub2).Multiple sclerosis (MS), a chronic inflammatory and neurodegenerative disease of the central nervous system (CNS), is characterized by recurrent relapses of CNS inflammation ranging from mild to severely disabling. Relapses have long been treated with steroids to reduce inflammation and hasten recovery. However, the commonly used intravenous methylprednisolone (IVMP) requires repeated infusions with the added costs of homecare or hospitalization, and may interfere with daily responsibilities. Oral steroids have been used in place of intravenous steroids, with lower direct and indirect costs.
Objectives: The primary objective was to compare efficacy of oral versus intravenous steroids in promoting disability recovery in MS relapses <= six weeks. Secondary objectives included subsequent relapse rate, disability, ambulation, hospitalization, immunological markers, radiological markers, and quality of life.
Search methods: A literature search was performed using Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group's Trials Register (January 2012), abstracts from meetings of the American Academy of Neurology (2008-2012), the European Federation of Neurological Sciences (2008-2012), the European Committee for Treatment and Research in Multiple Sclerosis and American Committee for Treatment and Research in Multiple Sclerosis (2008-2012) handsearching. No language restrictions were applied.
Selection criteria: Randomized or quasi-randomized trials comparing oral versus intravenous steroids for acute relapses (<= six weeks) in patients with clinically definite MSover age 16 were eligible.
Data collection and analysis: Three review authors (JB, PO and MH) participated in the independent assessment of all published articles as potentially relevant to the review. Any disagreement was resolved by discussion among review authors.We contacted study authors for additional information.Methodological quality was assessed by the same three review authors. Relevant data were extracted, and effect size was reported as mean difference (MD), mean difference (MD), odds ratio (OR) and absolute risk difference (ARD).
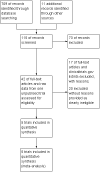
Main results: With this current update, a total of five eligible studies (215 patients) were identified. Only one outcome, the proportion of patients with Expanded Disability Status Scale (EDSS) improvement at four weeks, was common to three trials, while two trials examined magnetic resonance imaging (MRI) outcomes. The results of this review shows there is no significant difference in relapse recovery at week four (MD -0.22, 95% confidence interval (95% CI), 0.71 to 0.26, P = 0.20) nor differences in magnetic resonance imaging (MRI) gadolinium enhancement activity based on oral versus intravenous steroid treatment. However, only two of the five studies employed more current and rigorous methodological techniques, so these results must be taken with some caution. The Oral Megadose Corticosteroid Therapy of Acute Exacerbations of Multiple Sclerosis (OMEGA) trial and the "Efficacy and Safety of Methylprednisolone Per os Versus IV for the Treatment of Multiple Sclerosis (MS) Relapses" (COPOUSEP) trial, designed to address such limitations, are currently underway.
Authors' conclusions: The analysis of the five included trials comparing intravenous versus oral steroid therapy for MS relapses do not demonstrate any significant differences in clinical (benefits and adverse events), radiological or pharmacological outcomes. Based on the evidence, oral steroid therapy may be a practical and effective alternative to intravenous steroid therapy in the treatment of MS relapses.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures











































Update of
-
Oral versus intravenous steroids for treatment of relapses in multiple sclerosis.Cochrane Database Syst Rev. 2009 Jul 8;(3):CD006921. doi: 10.1002/14651858.CD006921.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2012 Dec 12;12:CD006921. doi: 10.1002/14651858.CD006921.pub3. PMID: 19588409 Updated.
References
References to studies included in this review
Alam 1993 {published data only}
Barnes 1997 {published and unpublished data}
-
- Barnes D, Hughes RAC, Morris RW, Wade‐Jones O, Brown P, Britton T, et al. Randomised trial of oral and intravenous methylprednisolone in acute relapses of multiple sclerosis. Lancet 1997;349:902‐6. - PubMed
-
- Pitzalis C, Sharrack B, Gray IA, Lee A, Hughes RAC. Comparison of the effects of oral versus intravenous methylprednisolone regimens on peripheral blood T lymphocyte adhesion molecule expression, T cell subsets distribution and TNF alpha concentrations in multiple sclerosis. Journal of Neuroimmunology 1997;74(1‐2):62‐8. - PubMed
-
- Sharrack B, Hughes RAC, Morris RW, Soudain S, Wade‐Jones O, Barnes D, et al. The effect of oral and intravenous methylprednisolone treatment of subsequent relapse rate in multiple sclerosis. Journal of the Neurological Sciences 2000;173:73‐7. - PubMed
Martinelli 2008 {published and unpublished data}
-
- Martinelli V, Pulizzi A, Annovazzi P, Rocca MA, Bucello S, Esposito F, et al. A single blind, randomised MRI study comparing high‐dose oral and intravenous methylprednisolone in treating MS relapses A single blind, randomised MRI study comparing high‐dose oral and intravenous methylprednisolone in treating MS relapses. Neurology 2009;73(22):1842‐1848. - PubMed
Morrow 2004 {published and unpublished data}
-
- Morrow SA, Stoian CA, Dmitrovic J, Chan SC, Metz LM. The bioavailability of iv methylprednisolone and oral prednisone in multiple sclerosis. Neurology 2004;63:1079‐80. - PubMed
Ramo‐Tello 2011 {unpublished data only}
-
- Ramo‐Tello C, Grau‐Lopez L, Giner P, Ramio‐Torrenta L, Brieva L, Saiz A, et al. A multicentre, randomized clinical and MRI study of highdose oral versus intravenous methylprednisolone in MS. Multiple Sclerosis 2011;17(10):Suppl 1 (S91‐S92).
References to studies excluded from this review
Alejandro 1994 {published data only}
-
- Alejandro PM, Castanon Gonzalez JA, Miranda Ruiz R, Edgar Echeverria R, Adriana Montano M. Comparative treatment of acute optic neuritis with "boluses" of intravenous methylprednisolone or oral prednisone. Gaceta Medica de Mexico 1994;130(4):227‐30. - PubMed
Beck 1992 {published data only}
-
- Beck RW, Cleary PA, Anderson MM Jr, Keltner JL, Shults WT, Kaufman DI, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. New England Journal of Medicine 1992;326(9):581‐8. - PubMed
De Keyser 1999 {published data only}
-
- Keyser J, Zwanikken CM, Zorgdrager A, Oenema D, Boon M. Treatment of acute relapses in multiple sclerosis at home with oral dexamethasone: a pilot study. Journal of Clinical Neuroscience 1999;6(5):382‐4. - PubMed
Demirkiran 1995 {published data only}
-
- Demirkiran M, Sarica Y, Karatas M, Bozdemir H. Efficacy of intravenous methylprednisolone and oral prednisone in multiple sclerosis. Annals of Medical Science 1995;4:91‐5.
Huen 1989 {published data only}
-
- Huen R, Emser W, Schimrigk S. Evoked potentials with intrathecal and systemic corticosteroid therapy in multiple sclerosis Evoked potentials with intrathecal and systemic corticosteroid therapy in multiple sclerosis. EEG‐EMG Zeitschrift für Elektroenzephalographie, Elektromyographie und verwandte Gebiete 1989;20(2):88‐91. - PubMed
La Mantia 1994 {published data only}
-
- Mantia L, Eoli M, Milanese C, Salmaggi A, Dufour A, Torri V. Double‐blind trial of dexamethasone versus methylprednisolone in multiple sclerosis acute relapses. European Neurology 1994;34(4):199‐203. - PubMed
Le Page 2007 {published data only}
-
- Page E, Veillard D, Lavat C, Edan G. Intravenous versus oral tolerance of methylprednisolone high doses in multiple sclerosis: an observational study of 97 patients. Multiple Sclerosis. 2007; Vol. 13:S176.
Metz 1999 {published data only}
-
- Metz LM, Sabuda D, Hilsden R, Enns R, Meddings JB. Gastric tolerance of high‐dose pulse oral prednisone in multiple sclerosis. Neurology 1999;53:2093. - PubMed
Milanese 1989 {published data only}
-
- Milanese C, Mantia L, Salmaggi A, Campi A, Eoli M, Scaioli V, et al. Double‐blind randomized trial of ACTH versus dexamethasone versus methylprednisolone in multiple sclerosis bouts. Clinical, cerebrospinal fluid and neurophysiological results. European Neurology 1989;29(1):10‐14. - PubMed
Murray 1999 {published data only}
Rohrbach 1988 {published data only}
-
- Rohrbach E, Kappos L, Stadt D, Hennes A. Effects, side‐effects and pharmacokinetics of intrathecal versus oral corticosteroids in spinal symptoms of multiple sclerosis: results of a double‐blind controlled trial. Journal of Neurology. 1988; Vol. 235:S40‐1. [CN‐00225511]
Sellebjerg 1998 {published data only}
-
- Sellebjerg F, Frederiksen JL, Nielsen PM, Olesen J. Double blind, randomized, placebo‐controlled study of oral, high‐dose methylprednisolone in attacks of MS. Neurology 1998;51(2):529‐34. - PubMed
Sellebjerg 1999 {published data only}
-
- Sellebjerg F, Nielsen HS, Frederiksen JL, Olesen J. A randomized, controlled trial of oral high‐dose methylprednisolone in acute optic neuritis. Neurology 1999;52(7):1479‐84. - PubMed
Tankisi 1997 {published data only}
-
- Tankisi H, Oztekin N, Oztekin MF, Ozbakir S, Guven H, Guven B. Relative efficacy of intravenous methylprednisolone and oral prednisolone in the treatment of acute relapse in multiple sclerosis. Multiple Sclerosis. 1997; Vol. Suppl 3:352. [CN‐00625757 (EMBASE)]
Thompson 1989 {published data only}
-
- Thompson AJ, Kennard C, Swash M, Summers B, Yuill GM, Shepherd DI, et al. Relative efficacy of intravenous methylprednisolone and ACTH in the treatment of acute relapse in MS. Neurology 1989;39(7):969‐71. - PubMed
Toczolowski 1995 {published data only}
-
- Toczolowski J, Lewandowska‐Furmanik M, Stelmasiak Z, Wozniak D, Chmiel M. Treatment of acute optic neuritis with large doses of corticosteroids. Klinika Oczna 1995;97:122‐5. - PubMed
Tourtellotte 1965 {published data only}
-
- Tourtellotte WW, Haerer AF. Use of an oral corticosteroid in the treatment of multiple sclerosis. A double ‐blind study. Archives of Neurology 1965;12:536‐45. - PubMed
References to ongoing studies
COPOUSEP 2009 {unpublished data only}
-
- Efficacy and Safety of Methylprednisolone Per os Versus IV for the Treatment of Multiple Sclerosis (MS) Relapses (COPOUSEP). Ongoing study March 2008.
OMEGA 2007 {unpublished data only}
-
- Lublin F. Oral megadose corticosteroid therapy of acute exacerbations of multiple sclerosis (OMEGA) NCT00418145. www.clinicaltrials.gov 2007.
Additional references
Andersson 1998
-
- Andersson P‐B, Goodkin DE. Glucocorticosteroid therapy for multiple sclerosis: a critical review. Journal of the Neurological Sciences 1998;160:16‐25. - PubMed
Brusaferri 2000
-
- Brusaferri F, Candelise L. Steroids for multiple sclerosis and optic neuritis: a meta‐analysis of randomized controlled clinical trials. Journal of Neurology 2000;247:435‐42. - PubMed
Confavreux 2006
-
- Confavreux C, Vukusic S. The natural history of multiple sclerosis. Revue du Practicien 2006;56(12):1313‐20. - PubMed
D'Amico 2007
-
- D'Amico R, Ebers G, Filippini G, Fredrikson S, Rice GPA, Simi S, et al. Cochrane Multiple Sclerosis Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2007, issue 2:Art.No.:MS.
Filippini 2000
Fisher 1999
-
- Fisher JS, Rudick RA, Cutter GR, Reingold SC for the National MS Society Clinical Outcomes Assessment Task Force. The Multiple Sclerosis Functional Composite measure (MSFC): an integrated approach to MS clinical outcome assessment. Multiple Sclerosis 1999;5(4):244‐50. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2011.
Jonsson 1951
-
- Jonsson B, Reis G, Sahlgren E. Experience of ACTH and cortisone treatment in some organic neurological cases. Acta Psychiatrica et Neurologica Scandinavica Supplementum 1951;74:60‐5. - PubMed
Kunz 1998
Kurtzke 1983
-
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33(11):1444‐52. - PubMed
McDonald 2001
-
- McDonald IW, Compston A, Edan G, Goodkin D, Hartung H‐P, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121‐7. - PubMed
Necela 2004
-
- Necela BM, Cidlowski JA. Mechanisms of glucocorticoid receptor action in noninflammatory and inflammatory cells. Proceedings of the American Thoracis Society 2004;1(3):239‐46. - PubMed
Noseworthy 2000
-
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. New England Journal of Medicine 2000;343(13):938‐52. - PubMed
O'Brien 2003
Polman 2005
-
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung H‐P, Kappos L, et al. Diagnostic criteria for multiple sclerosis:2005 revisions to the “McDonald Criteria”. Annals of Neurology 2005;58(6):840‐6. - PubMed
Poser 1983
-
- Poser CM, Paty DW, Scheinberg L, McDonald I, Davis SA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(3):227‐31. - PubMed
Rio 2006
-
- Río J, Nos C, Tintoré M, Téllez N, Galán I, Pelayo R, et al. Defining the response to interferon‐beta in relapsing‐remitting multiple sclerosis patients. Annals of Neurology 2006;59(2):344‐52. - PubMed
Robson 1998
-
- Robson LS, Bain C, Beck S, Guthrie S, Coyte PC, O'Connor P. Cost analysis of methylprednisolone treatment of multiple sclerosis patients. Canadian Journal of Neurological Sciences 1998;25(3):222‐9. - PubMed
Schumacher 1965
-
- Schumacker GA, Beebe G, Kibler RF, Kurland LT, Kurtzke JF, McDowell F, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Annals of the New York Academy of Science 1965;122:552–68. - PubMed
Schweingruber 2011
-
- Schweingruber A, Reichardt SD, Reichardt HM. Mechanisms of glucocorticoids in control of neuroinflammation. Journal of Neuroendocrinology 2011;24:174‐82. - PubMed
Sellebjerg 2000
-
- Sellebjerg F, Christiansen M, Jensen J, Frederiksen JL. Immunological effects of oral high‐dose methylprednisolone in acute optic neuritis and multiple sclerosis. European Journal of Neurology : the official journal of the European Federation of Neurological Societies 2000;7(3):281‐9. [PUBMED: 10886311] - PubMed
Swanton 2007
-
- Swanton JK, Rovira A, Tintore M, Altmann DR, Barkhof F, Filippi M, et al. MRI criteria for multiple sclerosis in patients presenting with clinically isolated syndromes: a multicentre retrospective study. Lancet Neurology 2007;6(8):677‐86. - PubMed
Tremlett 1998
Troiano 1985
-
- Troiano RA, Hafstein MP, Zito G, Ruderman MI, Dowling PC, Cook SD. The effect of oral corticosteroid dosage on CT enhancing multiple sclerosis plaques. Journal of the Neurological Sciences 1985;70:67‐72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

