Treatment for paraneoplastic neuropathies
- PMID: 23235647
- PMCID: PMC12067043
- DOI: 10.1002/14651858.CD007625.pub2
Treatment for paraneoplastic neuropathies
Abstract
Background: It is not unusual to observe peripheral nervous system involvement in people with tumours outside the nervous system. Any part of the peripheral nervous system can be involved, from sensory and motor neurons to nerve roots and plexuses, from distal trunks to neuromuscular junctions. Pathogenesis also varies from direct infiltration by cancer cells, to treatment toxicity, to metabolic derangement, cachexia, infections and paraneoplastic syndromes.Paraneoplastic neurological syndromes are symptoms or signs resulting from damage to organs or tissues that are remote from the site of the malignancy or its metastases. The pathogenesis is thought to be immune-mediated as a result of a cross-reaction against antigens shared by the tumour and nervous system cells.Paraneoplastic neuropathies are the most frequently reported paraneoplastic syndromes. They are, however, heterogeneous and require several therapeutic approaches. This review was undertaken to systematically assess any data available from randomised controlled trials (RCTs) on the treatment of paraneoplastic syndromes of the peripheral nervous system and not the whole range of paraneoplastic neurological syndromes.
Objectives: To assess the benefits and harms of treatments for paraneoplastic neuropathies.
Search methods: We searched the Cochrane Neuromuscular Disease Group Specialized Register (14 February 2012), CENTRAL (2012, Issue 1), MEDLINE (January 1966 to February 2012), EMBASE (January 1980 to February 2012) and LILACS (January 1982 to February 2012) for RCTs, quasi-RCTs, historically controlled studies and trials with concurrent controls.We adapted this strategy to search MEDLINE from 1966 and EMBASE from 1980 for comparative cohort studies, case-control studies and case series.
Selection criteria: We planned to include all RCTs and quasi-RCTs (in which allocation is not random but is intended to be unbiased, for example alternate allocation) of any treatment for paraneoplastic neuropathies. Since we expected there to be few or no included studies, we also planned to assess and summarise observational studies, prospective and retrospective comparative cohort studies, case-control studies and case series that met minimum criteria in the discussion.
Data collection and analysis: Three review authors selected the trials for inclusion. When there was any disagreement we reached an agreement by discussion. Two review authors extracted data independently onto a specially designed data extraction form. We would have collected adverse event data from included studies.
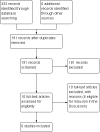
Main results: Despite many reports on paraneoplastic neuropathy, we identified no RCT or quasi-RCTs for inclusion in this review. We found only six studies, involving 54 participants, from among the non-randomised evidence that were judged by predefined criteria to be of suitable quality for inclusion in the discussion. These studies were not readily comparable. The treatments focused on tumour treatment and immunomodulation, mainly intravenous immunoglobulin.
Authors' conclusions: At present there are no RCTs or quasi-RCTs of treatment for paraneoplastic neuropathies on which to base practice. There is only evidence from case series, case reports or expert opinion (class IV evidence) for the effect of immunomodulation (intravenous immunoglobulin, plasma exchange, steroid treatment or chemotherapy) on paraneoplastic neuropathy.
Conflict of interest statement
W. Grisold is an European Association of Neuro‐Oncology (EANO) board member and a World Federation of Neurology (WFN) board member. He is also co‐author of an atlas of neuromuscular diseases. He has received consultancy fees from Biogen Idec, for work unrelated to neuropathies. He has no known conflict of interest.
Other authors: no known conflicts of interest.
Update of
- doi: 10.1002/14651858.CD007625
References
References to studies excluded from this review
Anderson 1988 {published data only}
-
- Anderson NE, Rosenblum MK, Graus F, Wiley RG, Posner JB. Autoantibodies in paraneoplastic syndromes associated with small‐cell lung cancer. Neurology 1988;38(9):1391‐8. [PUBMED: 2842702] - PubMed
Dalmau 1992 {published data only}
-
- Dalmau J, Graus F, Rosenblum MK, Posner JB. Anti‐Hu‐associated paraneoplastic encephalomyelitis/sensory neuronopathy. A clinical study of 71 patients. Medicine (Baltimore) 1992;71(2):59‐72. [PUBMED: 1312211] - PubMed
Graus 1994 {published data only}
-
- Graus F, Bonaventura I, Uchuya M, Valls‐Solé J, Reñé R, Leger JM, et al. Indolent anti‐Hu‐associated paraneoplastic sensory neuropathy. Neurology 1994;44(12):2258‐61. [PUBMED: 7991109] - PubMed
Graus 2001 {published data only}
-
- Graus F, Keime‐Guibert F, Reñe R, Benyahia B, Ribalta T, Ascaso C, et al. Anti‐Hu‐associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain 2001;124(Pt 6):1138‐48. [PUBMED: 11353730] - PubMed
Keime‐Guibert 2000 {published data only}
-
- Keime‐Guibert F, Graus F, Fleury A, Reñé R, Honnorat J, Broet P, et al. Treatment of paraneoplastic neurological syndromes with antineuronal antibodies (Anti‐Hu, anti‐Yo) with a combination of immunoglobulins, cyclophosphamide, and methylprednisolone. Journal of Neurology, Neurosurgery and Psychiatry 2000;68(4):479‐82. [PUBMED: 10727484] - PMC - PubMed
Peterson 1994 {published data only}
-
- Peterson K, Forsyth PA, Posner JB. Paraneoplastic sensorimotor neuropathy associated with breast cancer. Journal of Neuro‐Oncology 1994;21(2):159‐70. [PUBMED: 7861192] - PubMed
Sillevis Smitt 2002 {published data only}
-
- Sillevis Smitt P, Grefkens J, Leeuw B, Bent M, Putten W, Hooijkaas H, et al. Survival and outcome in 73 anti‐Hu positive patients with paraneoplastic encephalomyelitis/sensory neuronopathy. Journal of Neurology 2002;249(6):745‐53. [PUBMED: 12111309 ] - PubMed
Uchuya 1996 {published data only}
Valldeoriola 1992 {published data only}
-
- Valldeoriola F, Vega F, Reñé R, Pou A, Roquer J, Delattre JY, Graus F. Neurologic syndromes associated with anti‐Hu antibody. Study of 24 patients. Medicina Clinica 1992;99(10):361‐4. [PUBMED: 1334171] - PubMed
Viala 2008 {published data only}
-
- Viala K, Béhin A, Maisonobe T, Léger JM, Stojkovic T, Davi F, et al. Neuropathy in lymphoma: a relationship between the pattern of neuropathy, type of lymphoma and prognosis?. Journal of Neurology, Neurosurgery and Psychiatry 2008;79(7):778‐82. [PUBMED: 17971432] - PubMed
Additional references
Antoine 2007
-
- Antoine JC, Camdessanché JP. Peripheral nervous system involvement in patients with cancer. Lancet Neurology 2007;6(1):75‐86. - PubMed
Darnell 2003
-
- Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. NEJM 2003;349(16):1543‐54. - PubMed
Dyck 2005
-
- Dyck PJ, Hughes RAC, O'Brien PC. Quantitating overall neuropathic symptoms, impairments and outcomes. In: Dyck PJ, Thomas PK editor(s). Peripheral Neuropathy. 4th Edition. Philadelphia: Elsevier, 2005:1031‐50.
Giometto 1999
Graus 2004
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johnson 1979
-
- Johnson PC, Rolak LA, Hamilton RH, Laguna JF. Paraneoplastic vasculitis of nerve: a remote effect of cancer. Annals of Neurology 1979;5(5):437‐44. - PubMed
Oh 1979
-
- Oh SJ. Paraneoplastic vasculitis of the peripheral nervous system. Neurologic Clinics 1997;15(4):849‐63. - PubMed
Posner 1995
-
- Posner JB. Paraneoplastic syndromes. In: Posner JB editor(s). In: Neurologic Complications of Cancer. Vol. Contemporary Neurology Series 45, Philadelphia: FA Davis Company, 1995:353‐83.
Rankin 1957
-
- Rankin J. Cerebral vascular accidents in patients over the age of 60. II Prognosis. Scottish Medical Journal 1957;2(5):200‐15. - PubMed
Vigliani 2004
-
- Vigliani MC, Magistrello M, Polo P, Mutani R, Chio A. Risk of cancer in patients with Guillain‐Barré syndrome (GBS): a population‐based study. Journal of Neurology 2004;251:321‐6. - PubMed
Ware 1992
-
- Ware JE Jr, Sherbourne CD. The MOS 36‐item Short‐Form Health Survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous