LTP requires a reserve pool of glutamate receptors independent of subunit type
- PMID: 23235828
- PMCID: PMC3998843
- DOI: 10.1038/nature11775
LTP requires a reserve pool of glutamate receptors independent of subunit type
Abstract
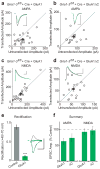
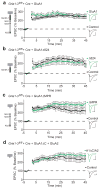
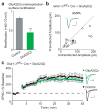
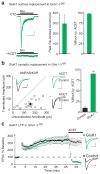
Long-term potentiation (LTP) of synaptic transmission is thought to be an important cellular mechanism underlying memory formation. A widely accepted model posits that LTP requires the cytoplasmic carboxyl tail (C-tail) of the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptor subunit GluA1. To find the minimum necessary requirement of the GluA1 C-tail for LTP in mouse CA1 hippocampal pyramidal neurons, we used a single-cell molecular replacement strategy to replace all endogenous AMPA receptors with transfected subunits. In contrast to the prevailing model, we found no requirement of the GluA1 C-tail for LTP. In fact, replacement with the GluA2 subunit showed normal LTP, as did an artificially expressed kainate receptor not normally found at these synapses. The only conditions under which LTP was impaired were those with markedly decreased AMPA receptor surface expression, indicating a requirement for a reserve pool of receptors. These results demonstrate the synapse's remarkable flexibility to potentiate with a variety of glutamate receptor subtypes, requiring a fundamental change in our thinking with regard to the core molecular events underlying synaptic plasticity.
Figures


Comment in
-
Synaptic plasticity: A twist in the LTP tail.Nat Rev Neurosci. 2013 Feb;14(2):80-1. doi: 10.1038/nrn3428. Epub 2012 Dec 28. Nat Rev Neurosci. 2013. PMID: 23271114 No abstract available.
-
Neuroscience: Strength in numbers.Nature. 2013 Jan 24;493(7433):482-3. doi: 10.1038/493482a. Nature. 2013. PMID: 23344353 Free PMC article.
References
-
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. - PubMed
-
- Wisden W, Seeburg PH. Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol. 1993;3:291–8. - PubMed
-
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

