Longitudinal in vivo monitoring of rodent glioma models through thinned skull using laser speckle contrast imaging
- PMID: 23235836
- PMCID: PMC3519490
- DOI: 10.1117/1.JBO.17.12.126017
Longitudinal in vivo monitoring of rodent glioma models through thinned skull using laser speckle contrast imaging
Abstract
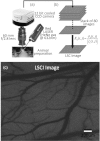
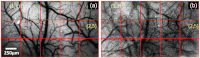
Laser speckle contrast imaging (LSCI) is a contrast agent free imaging technique suited for longitudinal assessment of vascular remodeling that accompanies brain tumor growth. We report the use of LSCI to monitor vascular changes in a rodent glioma model. Ten rats are inoculated with 9L gliosarcoma cells, and the angiogenic response is monitored five times over two weeks through a thinned skull imaging window. We are able to visualize neovascularization and measure the number of vessels per unit area to assess quantitatively the microvessel density (MVD). Spatial spread of MVD reveals regions of high MVD that may correspond to tumor location. Whole-field average MVD values increase with time in the tumor group but are fairly stable in the control groups. Statistical analysis shows significant differences in MVD values between the tumor group and both saline-receiving and unperturbed control groups over the two-week period (p<0.05). In conclusion, LSCI is suitable for investigation of tumor angiogenesis in rodent models. In addition, the statistical difference (p<0.02) between MVD values of the tumor (24.40 ± 1.41) and control groups (15.40 ± 1.60) on the 14th day after inoculation suggests a potential use of LSCI in the clinic in distinguishing tumor environments from normal vasculature.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

