Identification of novel influenza A virus proteins translated from PA mRNA
- PMID: 23236060
- PMCID: PMC3571384
- DOI: 10.1128/JVI.02656-12
Identification of novel influenza A virus proteins translated from PA mRNA
Abstract
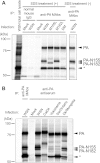
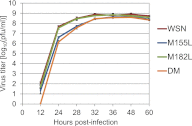
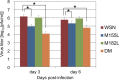
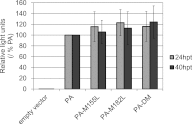
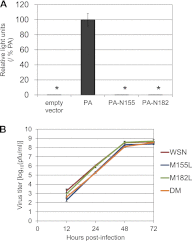
Many replication events are involved in the influenza A virus life cycle, and they are accomplished by different virus proteins with specific functions. However, because the size of the influenza virus genome is limited, the virus uses different mechanisms to express multiple viral proteins from a single gene segment. The M2 and NS2 proteins are produced by splicing, and several novel influenza A virus proteins, such as PB1-F2, PB1-N40, and PA-X, have recently been identified. Here, we identified novel PA-related proteins in influenza A virus-infected cells. These newly identified proteins are translated from the 11th and 13th in-frame AUG codons in the PA mRNA and are, therefore, N-terminally truncated forms of PA, which we named PA-N155 and PA-N182, respectively. The 11th and 13th AUG codons are highly conserved among influenza A viruses, and the PA-N155 and PA-N182 proteins were detected in cells infected with various influenza A viruses isolated from different host species, suggesting the expression of these N-truncated PAs is universal in nature among influenza A viruses. These N-truncated PAs did not show polymerase activity when expressed together with PB1 and PB2; however, mutant viruses lacking the N-truncated PAs replicated more slowly in cell culture and had lower pathogenicity in mice than did wild-type virus. These results suggest that these novel PA-related proteins likely possess important functions in the replication cycle of influenza A virus.
Figures

References
-
- Sugiura A. 1975. Introduction-historical review, p 171–213 In Kilbourne ED. (ed), The influenza viruses and influenza. Academic Press, New York, NY
-
- Palese P. 1977. The genes of influenza virus. Cell 10:1–10 - PubMed
-
- Lamb RA, Lai CJ. 1980. Sequence of interrupted and uninterrupted mRNAs and cloned DNA coding for the two overlapping nonstructural proteins of influenza virus. Cell 21:475–485 - PubMed
-
- Lamb RA. 1983. The influenza virus RNA segments and their encoded proteins, p 26–69 In Palese P, Kingsbury DW. (ed), Genetics of influenza viruses. Springer, New York, NY
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

