Signaling axis involving Hedgehog, Notch, and Scl promotes the embryonic endothelial-to-hematopoietic transition
- PMID: 23236128
- PMCID: PMC3545793
- DOI: 10.1073/pnas.1214361110
Signaling axis involving Hedgehog, Notch, and Scl promotes the embryonic endothelial-to-hematopoietic transition
Erratum in
-
Correction to Supporting Information for Kim et al., Signaling axis involving Hedgehog, Notch, and Scl promotes the embryonic endothelial-to-hematopoietic transition.Proc Natl Acad Sci U S A. 2025 Jul 15;122(28):e2516068122. doi: 10.1073/pnas.2516068122. Epub 2025 Jul 7. Proc Natl Acad Sci U S A. 2025. PMID: 40623200 Free PMC article. No abstract available.
Abstract
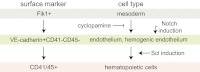
During development, the hematopoietic lineage transits through hemogenic endothelium, but the signaling pathways effecting this transition are incompletely characterized. Although the Hedgehog (Hh) pathway is hypothesized to play a role in patterning blood formation, early embryonic lethality of mice lacking Hh signaling precludes such analysis. To determine a role for Hh signaling in patterning of hemogenic endothelium, we assessed the effect of altered Hh signaling in differentiating mouse ES cells, cultured mouse embryos, and developing zebrafish embryos. In differentiating mouse ES cells and mouse yolk sac cultures, addition of Indian Hh ligand increased hematopoietic progenitors, whereas chemical inhibition of Hh signaling reduced hematopoietic progenitors without affecting primitive streak mesoderm formation. In the setting of Hh inhibition, induction of either Notch signaling or overexpression of Stem cell leukemia (Scl)/T-cell acute lymphocytic leukemia protein 1 rescued hemogenic vascular-endothelial cadherin(+) cells and hematopoietic progenitor formation. Together, our results reveal that Scl overexpression is sufficient to rescue the developmental defects caused by blocking the Hh and Notch pathways, and inform our understanding of the embryonic endothelial-to-hematopoietic transition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Moore MA, Metcalf D. Ontogeny of the haemopoietic system: Yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18(3):279–296. - PubMed
-
- Yoder MC, et al. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7(3):335–344. - PubMed
-
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86(6):897–906. - PubMed
-
- Kim PG, Daley GQ. Application of induced pluripotent stem cells to hematologic disease. Cytotherapy. 2009;11(8):980–989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

