Production of unique immunotoxin cancer therapeutics in algal chloroplasts
- PMID: 23236148
- PMCID: PMC3538218
- DOI: 10.1073/pnas.1214638110
Production of unique immunotoxin cancer therapeutics in algal chloroplasts
Abstract
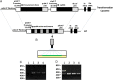
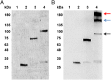
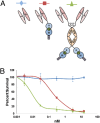
The idea of targeted therapy, whereby drug or protein molecules are delivered to specific cells, is a compelling approach to treating disease. Immunotoxins are one such targeted therapeutic, consisting of an antibody domain for binding target cells and molecules of a toxin that inhibits the proliferation of the targeted cell. One major hurdle preventing these therapies from reaching the market has been the lack of a suitable production platform that allows the cost-effective production of these highly complex molecules. The chloroplast of the green alga Chlamydomonas reinhardtii has been shown to contain the machinery necessary to fold and assemble complex eukaryotic proteins. However, the translational apparatus of chloroplasts resembles that of a prokaryote, allowing them to accumulate eukaryotic toxins that otherwise would kill a eukaryotic host. Here we show expression and accumulation of monomeric and dimeric immunotoxin proteins in algal chloroplasts. These fusion proteins contain an antibody domain targeting CD22, a B-cell surface epitope, and the enzymatic domain of exotoxin A from Pseudomonas aeruginosa. We demonstrated that algal-produced immunotoxins accumulate as soluble and enzymatically active proteins that bind target B cells and efficiently kill them in vitro. We also show that treatment with either the mono- or dimeric immunotoxins significantly prolongs the survival of mice with implanted human B-cell tumors.
Conflict of interest statement
Conflict of interest statement: S.P.M. is a founder of Sapphire Energy and has a financial interest in that company. Sapphire Energy has rights to this technology.
Figures




Similar articles
-
Production of anti-cancer immunotoxins in algae: ribosome inactivating proteins as fusion partners.Biotechnol Bioeng. 2013 Nov;110(11):2826-35. doi: 10.1002/bit.24966. Epub 2013 Jun 25. Biotechnol Bioeng. 2013. PMID: 23719862
-
Synthesis and assembly of a full-length human monoclonal antibody in algal chloroplasts.Biotechnol Bioeng. 2009 Nov 1;104(4):663-73. doi: 10.1002/bit.22446. Biotechnol Bioeng. 2009. PMID: 19562731
-
The algal chloroplast as a synthetic biology platform for production of therapeutic proteins.Microbiology (Reading). 2018 Feb;164(2):113-121. doi: 10.1099/mic.0.000599. Epub 2018 Jan 3. Microbiology (Reading). 2018. PMID: 29297850 Review.
-
Accumulation and processing of a recombinant protein designed as a cleavable fusion to the endogenous Rubisco LSU protein in Chlamydomonas chloroplast.BMC Biotechnol. 2009 Mar 26;9:26. doi: 10.1186/1472-6750-9-26. BMC Biotechnol. 2009. PMID: 19323825 Free PMC article.
-
Green biologics: The algal chloroplast as a platform for making biopharmaceuticals.Bioengineered. 2018 Jan 1;9(1):48-54. doi: 10.1080/21655979.2017.1377867. Epub 2017 Sep 29. Bioengineered. 2018. PMID: 28892417 Free PMC article. Review.
Cited by
-
Evaluation of Hydra HALT-1 as a toxin moiety for recombinant immunotoxin.BMC Biotechnol. 2020 Jun 17;20(1):31. doi: 10.1186/s12896-020-00628-9. BMC Biotechnol. 2020. PMID: 32552895 Free PMC article.
-
Plastid Transformation: How Does it Work? Can it Be Applied to Crops? What Can it Offer?Int J Mol Sci. 2020 Jul 9;21(14):4854. doi: 10.3390/ijms21144854. Int J Mol Sci. 2020. PMID: 32659946 Free PMC article. Review.
-
Current Status and Perspective on the Use of Viral-Based Vectors in Eukaryotic Microalgae.Mar Drugs. 2022 Jun 29;20(7):434. doi: 10.3390/md20070434. Mar Drugs. 2022. PMID: 35877728 Free PMC article. Review.
-
Targeted delivery of fluorogenic peptide aptamers into live microalgae by femtosecond laser photoporation at single-cell resolution.Sci Rep. 2018 May 29;8(1):8271. doi: 10.1038/s41598-018-26565-4. Sci Rep. 2018. PMID: 29844463 Free PMC article.
-
New tools for chloroplast genetic engineering allow the synthesis of human growth hormone in the green alga Chlamydomonas reinhardtii.Appl Microbiol Biotechnol. 2016 Jun;100(12):5467-77. doi: 10.1007/s00253-016-7354-6. Epub 2016 Feb 18. Appl Microbiol Biotechnol. 2016. PMID: 26887319 Free PMC article.
References
-
- Raghavendra AS. Photosynthesis: A Comprehensive Treatise. Cambridge, UK: Cambridge Univ Press; 1998.
-
- Tran M, Zhou B, Pettersson PL, Gonzalez MJ, Mayfield SP. Synthesis and assembly of a full-length human monoclonal antibody in algal chloroplasts. Biotechnol Bioeng. 2009;104(4):663–673. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

