Decoding the rules of recruitment of excitatory interneurons in the adult zebrafish locomotor network
- PMID: 23236181
- PMCID: PMC3535644
- DOI: 10.1073/pnas.1216256110
Decoding the rules of recruitment of excitatory interneurons in the adult zebrafish locomotor network
Abstract
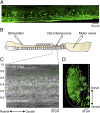
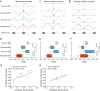
Neural networks in the spinal cord transform signals from the brain into coordinated locomotor movements. An optimal adjustment of the speed of locomotion entails a precise order of recruitment of interneurons underlying excitation within these networks. However, the mechanisms encoding the recruitment threshold of excitatory interneurons have remained unclear. Here we show, using a juvenile/adult zebrafish preparation, that excitatory V2a interneurons are incrementally recruited with increased swimming frequency. The order of recruitment is not imprinted by the topography or the input resistance of the V2a interneurons. Rather, it is determined by scaling the effect of excitatory synaptic currents by the input resistance. We also show that the locomotor networks are composed of multiple microcircuits encompassing subsets of V2a interneurons and motoneurons that are recruited in a continuum with increased swimming speeds. Thus, our results provide insights into the organization and mechanisms determining the recruitment of spinal microcircuits to ensure optimal execution of locomotor movements.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
Spinal cord: Speeding up locomotion.Nat Rev Neurosci. 2013 Feb;14(2):78-9. doi: 10.1038/nrn3435. Epub 2013 Jan 9. Nat Rev Neurosci. 2013. PMID: 23299060 No abstract available.
Similar articles
-
Differences in the morphology of spinal V2a neurons reflect their recruitment order during swimming in larval zebrafish.J Comp Neurol. 2014 Apr 15;522(6):1232-48. doi: 10.1002/cne.23465. J Comp Neurol. 2014. PMID: 24114934 Free PMC article.
-
Separate microcircuit modules of distinct v2a interneurons and motoneurons control the speed of locomotion.Neuron. 2014 Aug 20;83(4):934-43. doi: 10.1016/j.neuron.2014.07.018. Epub 2014 Aug 7. Neuron. 2014. PMID: 25123308
-
Frequency-dependent recruitment of V2a interneurons during fictive locomotion in the mouse spinal cord.Nat Commun. 2011;2:274. doi: 10.1038/ncomms1276. Nat Commun. 2011. PMID: 21505430 Free PMC article.
-
Principles Governing Locomotion in Vertebrates: Lessons From Zebrafish.Front Neural Circuits. 2018 Sep 13;12:73. doi: 10.3389/fncir.2018.00073. eCollection 2018. Front Neural Circuits. 2018. PMID: 30271327 Free PMC article. Review.
-
Origin and circuitry of spinal locomotor interneurons generating different speeds.Curr Opin Neurobiol. 2018 Dec;53:16-21. doi: 10.1016/j.conb.2018.04.024. Epub 2018 May 4. Curr Opin Neurobiol. 2018. PMID: 29733915 Review.
Cited by
-
V2a interneuron diversity tailors spinal circuit organization to control the vigor of locomotor movements.Nat Commun. 2018 Aug 22;9(1):3370. doi: 10.1038/s41467-018-05827-9. Nat Commun. 2018. PMID: 30135498 Free PMC article.
-
Molecular blueprints for spinal circuit modules controlling locomotor speed in zebrafish.Nat Neurosci. 2024 Jan;27(1):78-89. doi: 10.1038/s41593-023-01479-1. Epub 2023 Nov 2. Nat Neurosci. 2024. PMID: 37919423 Free PMC article.
-
Optogenetic activation of excitatory premotor interneurons is sufficient to generate coordinated locomotor activity in larval zebrafish.J Neurosci. 2014 Jan 1;34(1):134-9. doi: 10.1523/JNEUROSCI.4087-13.2014. J Neurosci. 2014. PMID: 24381274 Free PMC article.
-
Decoding the organization of spinal circuits that control locomotion.Nat Rev Neurosci. 2016 Apr;17(4):224-38. doi: 10.1038/nrn.2016.9. Epub 2016 Mar 3. Nat Rev Neurosci. 2016. PMID: 26935168 Free PMC article. Review.
-
A Mammalian Retinal Ganglion Cell Implements a Neuronal Computation That Maximizes the SNR of Its Postsynaptic Currents.J Neurosci. 2017 Feb 8;37(6):1468-1478. doi: 10.1523/JNEUROSCI.2814-16.2016. Epub 2016 Dec 30. J Neurosci. 2017. PMID: 28039376 Free PMC article.
References
-
- Büschges A, Scholz H, El Manira A. New moves in motor control. Curr Biol. 2011;21(13):R513–R524. - PubMed
-
- Grillner S. The motor infrastructure: From ion channels to neuronal networks. Nat Rev Neurosci. 2003;4(7):573–586. - PubMed
-
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

