Role of the prefrontal cortex in altered hippocampal-accumbens synaptic plasticity in a developmental animal model of schizophrenia
- PMID: 23236209
- PMCID: PMC4047286
- DOI: 10.1093/cercor/bhs380
Role of the prefrontal cortex in altered hippocampal-accumbens synaptic plasticity in a developmental animal model of schizophrenia
Abstract
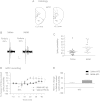
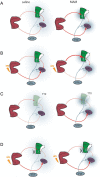
Schizophrenia is characterized by alterations in cortico-limbic processes believed to involve modifications in activity within the prefrontal cortex (PFC) and the hippocampus. The nucleus accumbens (NAc) integrates information from these 2 brain regions and is involved in cognitive and psychomotor functions that are disrupted in schizophrenia, indicating an important role for this structure in the pathophysiology of this disorder. In this study, we used in vivo electrophysiological recordings from the NAc and the PFC of adult rats and the MAM developmental disruption rodent model of schizophrenia to explore the influence of the medial PFC on the hippocampal-accumbens pathway. We found that, in MAM-treated rats, tetanization of hippocampal inputs to the NAc produce opposite synaptic plasticity compared with controls, which is a consequence of alterations in the hippocampal-mPFC pathway. Moreover, we show that administration of the D2-receptor-blocking antipsychotic drug sulpiride either systemically or directly into the mPFC reverses the alterations in the MAM rat. Therefore, specific disruptions in cortical and hippocampal inputs in the MAM-treated rat abnormally alter plasticity in subcortical structures. Moreover, our results suggest that, in the presence of antipsychotic drugs, the disrupted plasticities are normalized, supporting a role for this mechanism in antipsychotic drug action in schizophrenia.
Keywords: dopamine; long-term potentiation; nucleus accumbens; prefrontal cortex; schizophrenia.
Figures




References
-
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–1589. - PubMed
-
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. - PubMed
-
- Flagstad P, Mork A, Glenthoj BY, van Beek J, Michael-Titus AT, Didriksen M. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology. 2004;29:2052–2064. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

