Impact of tumor microenvironment and epithelial phenotypes on metabolism in breast cancer
- PMID: 23236214
- PMCID: PMC3684709
- DOI: 10.1158/1078-0432.CCR-12-2123
Impact of tumor microenvironment and epithelial phenotypes on metabolism in breast cancer
Abstract
Purpose: Cancer cells have altered metabolism, with increased glucose uptake, glycolysis, and biomass production. This study conducted genomic and metabolomic analyses to elucidate how tumor and stromal genomic characteristics influence tumor metabolism.
Experimental design: Thirty-three breast tumors and six normal breast tissues were analyzed by gene expression microarray and by mass spectrometry for metabolites. Gene expression data and clinical characteristics were evaluated in association with metabolic phenotype. To evaluate the role of stromal interactions in altered metabolism, cocultures were conducted using breast cancer cells and primary cancer-associated fibroblasts (CAF).
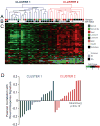
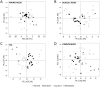
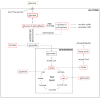
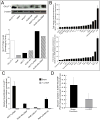
Results: Across all metabolites, unsupervised clustering resulted in two main sample clusters. Normal breast tissue and a subset of tumors with less aggressive clinical characteristics had lower levels of nucleic and amino acids and glycolysis byproducts, whereas more aggressive tumors had higher levels of these Warburg-associated metabolites. While tumor-intrinsic subtype did not predict metabolic phenotype, metabolic cluster was significantly associated with expression of a wound response signature. In cocultures, CAFs from basal-like breast cancers increased glucose uptake and basal-like epithelial cells increased glucose oxidation and glycogen synthesis, suggesting interplay of stromal and epithelial phenotypes on metabolism. Cytokine arrays identified hepatocyte growth factor (HGF) as a potential mediator of stromal-epithelial interaction and antibody neutralization of HGF resulted in reduced expression of glucose transporter 1 (GLUT1) and decreased glucose uptake by epithelium.
Conclusions: Both tumor/epithelial and stromal characteristics play important roles in metabolism. Warburg-like metabolism is influenced by changes in stromal-epithelial interactions, including altered expression of HGF/Met pathway and GLUT1 expression.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- K99 AA017376/AA/NIAAA NIH HHS/United States
- P30DK056350/DK/NIDDK NIH HHS/United States
- R00 AA017376/AA/NIAAA NIH HHS/United States
- R01 CA138255/CA/NCI NIH HHS/United States
- AA017376/AA/NIAAA NIH HHS/United States
- R01-CA138255/CA/NCI NIH HHS/United States
- U01 ES019472/ES/NIEHS NIH HHS/United States
- P30 DK056350/DK/NIDDK NIH HHS/United States
- L30 CA130535/CA/NCI NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- U01-ES019472/ES/NIEHS NIH HHS/United States
- ES019472/ES/NIEHS NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- P50 CA058223/CA/NCI NIH HHS/United States
- P50-CA58223/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

