UGT1A1 predicts outcome in colorectal cancer treated with irinotecan and fluorouracil
- PMID: 23236239
- PMCID: PMC3516223
- DOI: 10.3748/wjg.v18.i45.6635
UGT1A1 predicts outcome in colorectal cancer treated with irinotecan and fluorouracil
Abstract
Aim: To evaluate effects of UDP-glucuronosyltransferase1A1 (UGT1A1) and thymidylate synthetase (TS) gene polymorphisms on irinotecan in metastatic colorectal cancer (mCRC).
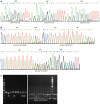
Methods: Two irinotecan- and fluorouracil-based regimens, FOLFIRI and IFL, were selected as second-line therapy for 138 Chinese mCRC patients. Genomic DNA was extracted from peripheral blood samples before treatment. UGT1A1 and TS gene polymorphisms were determined by direct sequencing and restriction fragment length polymorphism, respectively. Gene polymorphisms of UGT1A1*28, UGT1A1*6 and promoter enhancer region of TS were analyzed. The relationship between genetic polymorphisms and clinical outcome, that is, response, toxicity and survival were assessed. Pharmacokinetic analyses were performed in a subgroup patients based on different UGT1A1 genotypes. Plasma concentration of irinotecan and its active metabolite SN-38 and inactive metabolite SN-38G were determined by high performance liquid chromatography. Differences in irinotecan and its metabolites between UGT1A1 gene variants were compared.
Results: One hundred and eight patients received the FOLFIRI regimen, 29 the IFL regimen, and one irinotecan monotherapy. One hundred and thirty patients were eligible for toxicity and 111 for efficacy evaluation. One hundred and thirty-six patients were tested for UGT1A1*28 and *6 genotypes and 125 for promoter enhancer region of TS. Patients showed a higher frequency of wild-type UGT1A1*28 (TA6/6) compared with a Caucasian population (69.9% vs 45.2%). No significant difference was found between response rates and UGT1A1 genotype, although wild-type showed lower response rates compared with other variants (17.9% vs 24.2% for UGT1A1*28, 15.7% vs 26.8% for UGT1A1*6). When TS was considered, the subgroup with homozygous UGT1A1*28 (TA7/7) and non-3RG genotypes showed the highest response rate (33.3%), while wild-type UGT1A1*28 (TA6/6) with non-3RG only had a 13.6% response rate, but no significant difference was found. Logistic regression showed treatment duration was closely linked to clinical response. In toxicity comparison, UGT1A1*28 TA6/6 was associated with lower incidence of grade 2-4 diarrhea (27.8% vs 100%), and significantly reduced the risk of grade 4 neutropenia compared with TA7/7 (7.8% vs 37.5%). Wild-type UGT1A1*6 (G/G) tended to have a lower incidence of grade 3/4 diarrhea vs homozygous mutant (A/A) genotype (13.0% vs 40.0%). Taking UGT1A1 and TS genotypes together, lower incidence of grade 2-4 diarrhea was found in patients with non-3RG TS genotypes, when TA6/6 was compared with TA7/7 (35.3% vs 100.0%). No significant association with time to progression (TTP) and overall survival (OS) was observed with either UGT1A1 or TS gene polymorphisms, although slightly longer TTP and OS were found with UGT1A1*28 (TA6/6). Irinotecan PK was investigated in 34 patients, which showed high area under concentration curve (AUC) of irinotecan and SN-38, but low AUC ratio (SN-38G / SN-38) in those patients with UGT1A1*28 TA7/7.
Conclusion: A distinct distribution pattern of UGT1A1 genotypes in Chinese patients might contribute to relatively low toxicity associated with irinotecan and 5-fluorouracil in mCRC patients.
Keywords: Fluorouracil; Irinotecan; Metastatic colorectal cancer; Pharmacokinetics; Polymorphisms; Thymidylate synthetase; Toxicity; Treatment outcome; UDP-glucuronosyltransferase1A1.
Figures


Comment in
-
Therapeutic Application of Pharmacogenomics in Oncology.AAPS J. 2016 Jul;18(4):819-29. doi: 10.1208/s12248-016-9926-x. Epub 2016 May 13. AAPS J. 2016. PMID: 27178043
Similar articles
-
Correlation between UGT1A1 gene polymorphism and irinotecan chemotherapy in metastatic colorectal cancer: a study from Guangxi Zhuang.BMC Gastroenterol. 2020 Apr 7;20(1):96. doi: 10.1186/s12876-020-01227-w. BMC Gastroenterol. 2020. PMID: 32264830 Free PMC article.
-
The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer.J Clin Oncol. 2006 Jul 1;24(19):3061-8. doi: 10.1200/JCO.2005.05.5400. J Clin Oncol. 2006. PMID: 16809730 Clinical Trial.
-
UGT1A1*28 polymorphism predicts irinotecan-induced severe toxicities without affecting treatment outcome and survival in patients with metastatic colorectal carcinoma.Cancer. 2008 May 1;112(9):1932-40. doi: 10.1002/cncr.23370. Cancer. 2008. PMID: 18300238
-
Can UGT1A1 genotyping reduce morbidity and mortality in patients with metastatic colorectal cancer treated with irinotecan? An evidence-based review.Genet Med. 2009 Jan;11(1):21-34. doi: 10.1097/GIM.0b013e31818efd77. Genet Med. 2009. PMID: 19125129 Free PMC article. Review.
-
UGT1A1 genotyping: a predictor of irinotecan-associated side effects and drug efficacy?Anticancer Drugs. 2009 Nov;20(10):867-79. doi: 10.1097/CAD.0b013e328330c7d2. Anticancer Drugs. 2009. PMID: 19770637 Review.
Cited by
-
Predictive effects of bilirubin on response of colorectal cancer to irinotecan-based chemotherapy.World J Gastroenterol. 2016 Apr 28;22(16):4250-8. doi: 10.3748/wjg.v22.i16.4250. World J Gastroenterol. 2016. PMID: 27122675 Free PMC article. Clinical Trial.
-
The role of UGT1A1 polymorphism in the management of colorectal cancer.Oncol Rev. 2025 May 13;19:1547904. doi: 10.3389/or.2025.1547904. eCollection 2025. Oncol Rev. 2025. PMID: 40432911 Free PMC article. Review.
-
Computational Identification of Key Regulators in Two Different Colorectal Cancer Cell Lines.Front Genet. 2016 Apr 5;7:42. doi: 10.3389/fgene.2016.00042. eCollection 2016. Front Genet. 2016. PMID: 27092172 Free PMC article.
-
A rare case of primary choriocarcinoma in the sigmoid colon.World J Gastroenterol. 2013 Oct 21;19(39):6683-8. doi: 10.3748/wjg.v19.i39.6683. World J Gastroenterol. 2013. PMID: 24151399 Free PMC article.
-
Absolute quantification of UGT1A1 in various tissues and cell lines using isotope label-free UPLC-MS/MS method determines its turnover number and correlates with its glucuronidation activities.J Pharm Biomed Anal. 2014 Jan;88:180-90. doi: 10.1016/j.jpba.2013.08.024. Epub 2013 Aug 29. J Pharm Biomed Anal. 2014. PMID: 24055854 Free PMC article.
References
-
- Wang Y, Xu JM, Shen L, Xu N, Wang JW, Jiao SC, Zhang JS, Song ST, Li J, Bao HY, et al. Polymorphisms of UGT1A gene and irinotecan toxicity in Chinese colorectal cancer patients. Zhonghua Zhongliu Zazhi. 2007;29:913–916. - PubMed
-
- Braun MS, Richman SD, Quirke P, Daly C, Adlard JW, Elliott F, Barrett JH, Selby P, Meade AM, Stephens RJ, et al. Predictive biomarkers of chemotherapy efficacy in colorectal cancer: results from the UK MRC FOCUS trial. J Clin Oncol. 2008;26:2690–2698. - PubMed
-
- Di Paolo A, Bocci G, Polillo M, Del Re M, Di Desidero T, Lastella M, Danesi R. Pharmacokinetic and pharmacogenetic predictive markers of irinotecan activity and toxicity. Curr Drug Metab. 2011;12:932–943. - PubMed
-
- Fujita K, Ando Y, Nagashima F, Yamamoto W, Eodo H, Araki K, Kodama K, Miya T, Narabayashi M, Sasaki Y. Genetic linkage of UGT1A7 and UGT1A9 polymorphisms to UGT1A1*6 is associated with reduced activity for SN-38 in Japanese patients with cancer. Cancer Chemother Pharmacol. 2007;60:515–522. - PubMed
-
- Toffoli G, Cecchin E, Corona G, Russo A, Buonadonna A, D’Andrea M, Pasetto LM, Pessa S, Errante D, De Pangher V, et al. The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J Clin Oncol. 2006;24:3061–3068. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

