Recessive mutations in SPTBN2 implicate β-III spectrin in both cognitive and motor development
- PMID: 23236289
- PMCID: PMC3516553
- DOI: 10.1371/journal.pgen.1003074
Recessive mutations in SPTBN2 implicate β-III spectrin in both cognitive and motor development
Abstract
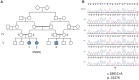
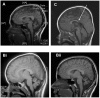
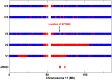
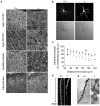
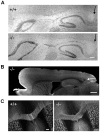
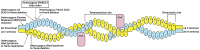
β-III spectrin is present in the brain and is known to be important in the function of the cerebellum. Heterozygous mutations in SPTBN2, the gene encoding β-III spectrin, cause Spinocerebellar Ataxia Type 5 (SCA5), an adult-onset, slowly progressive, autosomal-dominant pure cerebellar ataxia. SCA5 is sometimes known as "Lincoln ataxia," because the largest known family is descended from relatives of the United States President Abraham Lincoln. Using targeted capture and next-generation sequencing, we identified a homozygous stop codon in SPTBN2 in a consanguineous family in which childhood developmental ataxia co-segregates with cognitive impairment. The cognitive impairment could result from mutations in a second gene, but further analysis using whole-genome sequencing combined with SNP array analysis did not reveal any evidence of other mutations. We also examined a mouse knockout of β-III spectrin in which ataxia and progressive degeneration of cerebellar Purkinje cells has been previously reported and found morphological abnormalities in neurons from prefrontal cortex and deficits in object recognition tasks, consistent with the human cognitive phenotype. These data provide the first evidence that β-III spectrin plays an important role in cortical brain development and cognition, in addition to its function in the cerebellum; and we conclude that cognitive impairment is an integral part of this novel recessive ataxic syndrome, Spectrin-associated Autosomal Recessive Cerebellar Ataxia type 1 (SPARCA1). In addition, the identification of SPARCA1 and normal heterozygous carriers of the stop codon in SPTBN2 provides insights into the mechanism of molecular dominance in SCA5 and demonstrates that the cell-specific repertoire of spectrin subunits underlies a novel group of disorders, the neuronal spectrinopathies, which includes SCA5, SPARCA1, and a form of West syndrome.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Perrotta S, Gallagher PG, Mohandas N (2008) Hereditary spherocytosis. Lancet 372: 1411–1426. - PubMed
-
- Baines AJ (2010) The spectrin-ankyrin-4.1-adducin membrane skeleton: adapting eukaryotic cells to the demands of animal life. Protoplasma 244: 99–131. - PubMed
-
- Goodman SR, Zimmer WE, Clark MB, Zagon IS, Barker JE, et al. (1995) Brain spectrin: of mice and men. Brain Res Bull 36: 593–606. - PubMed
-
- Ikeda Y, Dick KA, Weatherspoon MR, Gincel D, Armbrust KR, et al. (2006) Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet 38: 184–190. - PubMed
-
- Nee LE, Higgins JJ (1997) Should spinocerebellar ataxia type 5 be called Lincoln ataxia? Neurology 49: 298–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

