Impact of anthelminthic treatment in pregnancy and childhood on immunisations, infections and eczema in childhood: a randomised controlled trial
- PMID: 23236367
- PMCID: PMC3517620
- DOI: 10.1371/journal.pone.0050325
Impact of anthelminthic treatment in pregnancy and childhood on immunisations, infections and eczema in childhood: a randomised controlled trial
Abstract
Background: Helminth infections may modulate immune responses to unrelated pathogens and allergens; these effects may commence prenatally. We addressed the hypothesis that anthelminthic treatment in pregnancy and early childhood would improve responses to immunisation and modulate disease incidence in early childhood with both beneficial and detrimental effects.
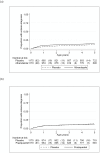
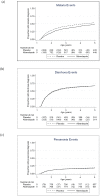
Methods and findings: A randomised, double-blind, placebo-controlled trial was conducted in Entebbe, Uganda [ISRCTN32849447]. In three independent randomisations, 2507 pregnant women were allocated to receive single-dose albendazole or placebo, and praziquantel or placebo; 2016 of their offspring were randomised to receive quarterly single-dose albendazole or placebo from age 15 months to 5 years. Primary outcomes were post-immunisation recall responses to BCG and tetanus antigens, and incidence of malaria, diarrhoea, and pneumonia; incidence of eczema was an important secondary outcome. Analysis was by intention-to-treat. Of 2345 live births, 1622 (69%) children remained in follow-up at age 5 years. 68% of mothers at enrolment, and 11% of five-year-olds, had helminth infections. Maternal hookworm and Schistosoma mansoni were effectively treated by albendazole and praziquantel, respectively; and childhood hookworm and Ascaris by quarterly albendazole. Incidence rates of malaria, diarrhoea, pneumonia, and eczema were 34, 65, 10 and 5 per 100 py, respectively. Albendazole during pregnancy caused an increased rate of eczema in the children (HR 1.58 (95% CI 1.15-2.17), p = 0.005). Quarterly albendazole during childhood was associated with reduced incidence of clinical malaria (HR 0.85 (95% CI 0.73-0.98), p = 0.03). There were no consistent effects of the interventions on any other outcome.
Conclusions: Routine use of albendazole in pregnancy may not always be beneficial, even in tropical developing countries. By contrast, regular albendazole treatment in preschool children may have an additional benefit for malaria control where helminths and malaria are co-endemic. Given the low helminth prevalence in our children, the effect of albendazole on malaria is likely to be direct.
Trial registration: Current Controlled Trials ISRCTN32849447.
Conflict of interest statement
Figures
References
-
- WHO (2002) Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. Geneva: World Health Organization. - PubMed
-
- de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, et al. (2003) Soil-transmitted helminth infections: updating the global picture. Trends Parasitol 19: 547–551. - PubMed
-
- WHO (2011) World Health Statistics. Geneva: World Health Organisation.
-
- WHO (2009) Global tuberculosis control - epidemiology, strategy, financing. WHO/HTM/TB/2009411.
-
- Fine PE (1995) Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346: 1339–1345. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

