Anatomical brain images alone can accurately diagnose chronic neuropsychiatric illnesses
- PMID: 23236384
- PMCID: PMC3517530
- DOI: 10.1371/journal.pone.0050698
Anatomical brain images alone can accurately diagnose chronic neuropsychiatric illnesses
Abstract
Objective: Diagnoses using imaging-based measures alone offer the hope of improving the accuracy of clinical diagnosis, thereby reducing the costs associated with incorrect treatments. Previous attempts to use brain imaging for diagnosis, however, have had only limited success in diagnosing patients who are independent of the samples used to derive the diagnostic algorithms. We aimed to develop a classification algorithm that can accurately diagnose chronic, well-characterized neuropsychiatric illness in single individuals, given the availability of sufficiently precise delineations of brain regions across several neural systems in anatomical MR images of the brain.
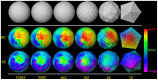
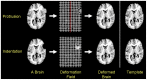
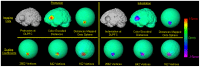
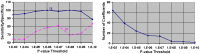
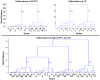
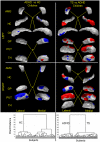
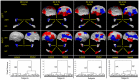
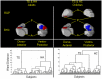
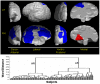
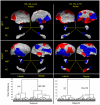
Methods: We have developed an automated method to diagnose individuals as having one of various neuropsychiatric illnesses using only anatomical MRI scans. The method employs a semi-supervised learning algorithm that discovers natural groupings of brains based on the spatial patterns of variation in the morphology of the cerebral cortex and other brain regions. We used split-half and leave-one-out cross-validation analyses in large MRI datasets to assess the reproducibility and diagnostic accuracy of those groupings.
Results: In MRI datasets from persons with Attention-Deficit/Hyperactivity Disorder, Schizophrenia, Tourette Syndrome, Bipolar Disorder, or persons at high or low familial risk for Major Depressive Disorder, our method discriminated with high specificity and nearly perfect sensitivity the brains of persons who had one specific neuropsychiatric disorder from the brains of healthy participants and the brains of persons who had a different neuropsychiatric disorder.
Conclusions: Although the classification algorithm presupposes the availability of precisely delineated brain regions, our findings suggest that patterns of morphological variation across brain surfaces, extracted from MRI scans alone, can successfully diagnose the presence of chronic neuropsychiatric disorders. Extensions of these methods are likely to provide biomarkers that will aid in identifying biological subtypes of those disorders, predicting disease course, and individualizing treatments for a wide range of neuropsychiatric illnesses.
Conflict of interest statement
Figures