Structural characterization of the interaction of human lactoferrin with calmodulin
- PMID: 23236421
- PMCID: PMC3516504
- DOI: 10.1371/journal.pone.0051026
Structural characterization of the interaction of human lactoferrin with calmodulin
Abstract
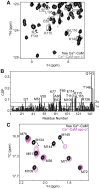
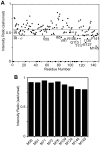
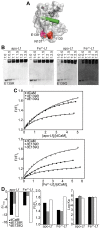
Lactoferrin (Lf) is an 80 kDa, iron (Fe(3+))-binding immunoregulatory glycoprotein secreted into most exocrine fluids, found in high concentrations in colostrum and milk, and released from neutrophil secondary granules at sites of infection and inflammation. In a number of cell types, Lf is internalized through receptor-mediated endocytosis and targeted to the nucleus where it has been demonstrated to act as a transcriptional trans-activator. Here we characterize human Lf's interaction with calmodulin (CaM), a ubiquitous, 17 kDa regulatory calcium (Ca(2+))-binding protein localized in the cytoplasm and nucleus of activated cells. Due to the size of this intermolecular complex (∼100 kDa), TROSY-based NMR techniques were employed to structurally characterize Ca(2+)-CaM when bound to intact apo-Lf. Both CaM's backbone amides and the ε-methyl group of key methionine residues were used as probes in chemical shift perturbation and cross-saturation experiments to define the binding interface of apo-Lf on Ca(2+)-CaM. Unlike the collapsed conformation through which Ca(2+)-CaM binds the CaM-binding domains of its classical targets, Ca(2+)-CaM assumes an extended structure when bound to apo-Lf. Apo-Lf appears to interact predominantly with the C-terminal lobe of Ca(2+)-CaM, enabling the N-terminal lobe to potentially bind another target. Our use of intact apo-Lf has made possible the identification of a secondary interaction interface, removed from CaM's primary binding domain. Secondary interfaces play a key role in the target's response to CaM binding, highlighting the importance of studying intact complexes. This solution-based approach can be applied to study other regulatory calcium-binding EF-hand proteins in intact intermolecular complexes.
Conflict of interest statement
Figures


Similar articles
-
Endocytosis and degradation of bovine apo- and holo-lactoferrin by isolated rat hepatocytes are mediated by recycling calcium-dependent binding sites.Biochemistry. 1993 Dec 14;32(49):13749-60. doi: 10.1021/bi00212a046. Biochemistry. 1993. PMID: 8257710
-
Site-specific modification of calmodulin Ca²(+) affinity tunes the skeletal muscle ryanodine receptor activation profile.Biochem J. 2010 Nov 15;432(1):89-99. doi: 10.1042/BJ20100505. Biochem J. 2010. PMID: 20815817
-
Fast methionine-based solution structure determination of calcium-calmodulin complexes.J Biomol NMR. 2011 May;50(1):71-81. doi: 10.1007/s10858-011-9495-3. Epub 2011 Mar 1. J Biomol NMR. 2011. PMID: 21360154
-
Molecular mechanisms of calmodulin's functional versatility.Biochem Cell Biol. 1998;76(2-3):313-23. doi: 10.1139/bcb-76-2-3-313. Biochem Cell Biol. 1998. PMID: 9923700 Review.
-
The Merck Frosst Award Lecture 1994. Calmodulin: a versatile calcium mediator protein.Biochem Cell Biol. 1994 Sep-Oct;72(9-10):357-76. Biochem Cell Biol. 1994. PMID: 7605608 Review.
Cited by
-
Development of Teleost Intermuscular Bones Undergoing Intramembranous Ossification Based on Histological-Transcriptomic-Proteomic Data.Int J Mol Sci. 2019 Sep 22;20(19):4698. doi: 10.3390/ijms20194698. Int J Mol Sci. 2019. PMID: 31546739 Free PMC article.
-
Neurogranin alters the structure and calcium binding properties of calmodulin.J Biol Chem. 2014 May 23;289(21):14644-55. doi: 10.1074/jbc.M114.560656. Epub 2014 Apr 8. J Biol Chem. 2014. PMID: 24713697 Free PMC article.
-
Aridity modulates grassland biomass responses to combined drought and nutrient addition.Nat Ecol Evol. 2025 Jun;9(6):937-946. doi: 10.1038/s41559-025-02705-8. Epub 2025 May 19. Nat Ecol Evol. 2025. PMID: 40389741
-
Ceruloplasmin Reduces the Lactoferrin/Oleic Acid Antitumor Complex-Mediated Release of Heme-Containing Proteins from Blood Cells.Int J Mol Sci. 2023 Nov 24;24(23):16711. doi: 10.3390/ijms242316711. Int J Mol Sci. 2023. PMID: 38069040 Free PMC article.
-
Screening the anti infectivity potentials of native N- and C-lobes derived from the camel lactoferrin against hepatitis C virus.BMC Complement Altern Med. 2014 Jul 3;14:219. doi: 10.1186/1472-6882-14-219. BMC Complement Altern Med. 2014. PMID: 24993815 Free PMC article.
References
-
- Legrand D, Mazurier J (2010) A critical review of the roles of host lactoferrin in immunity. Biometals 23: 365–376. - PubMed
-
- Vogel HJ (2012) Lactoferrin, a bird’s eye view. Biochem Cell Biol 90: 233–244. - PubMed
-
- Baker HM, Baker EN (2012) A structural perspective on lactoferrin function. Biochem Cell Biol 90: 320–328. - PubMed
-
- Jenssen H, Hancock RE (2009) Antimicrobial properties of lactoferrin. Biochimie 91: 19–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

