Novel potential interacting partners of fibronectin in spontaneous animal model of interstitial cystitis
- PMID: 23236492
- PMCID: PMC3517491
- DOI: 10.1371/journal.pone.0051391
Novel potential interacting partners of fibronectin in spontaneous animal model of interstitial cystitis
Abstract
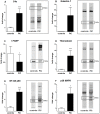
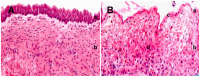
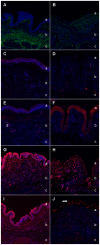
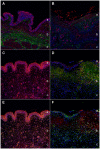
Feline idiopathic cystitis (FIC) is the only spontaneous animal model for human interstitial cystitis (IC), as both possess a distinctive chronical and relapsing character. Underlying pathomechanisms of both diseases are not clearly established yet. We recently detected increased urine fibronectin levels in FIC cases. The purpose of this study was to gain further insight into the pathogenesis by assessing interacting partners of fibronectin in urine of FIC affected cats. Several candidate proteins were identified via immunoprecipitation and mass spectrometry. Considerable changes in FIC conditions compared to physiological expression of co-purified proteins were detected by Western blot and immunohistochemistry. Compared to controls, complement C4a and thioredoxin were present in higher levels in urine of FIC patients whereas loss of signal intensity was detected in FIC affected tissue. Galectin-7 was exclusively detected in urine of FIC cats, pointing to an important role of this molecule in FIC pathogenesis. Moderate physiological signal intensity of galectin-7 in transitional epithelium shifted to distinct expression in transitional epithelium under pathophysiological conditions. I-FABP expression was reduced in urine and urinary bladder tissue of FIC cats. Additionally, transduction molecules of thioredoxin, NF-κB p65 and p38 MAPK, were examined. In FIC affected tissue, colocalization of thioredoxin and NF-κB p65 could be demonstrated compared to absent coexpression of thioredoxin and p38 MAPK. These considerable changes in expression level and pattern point to an important role for co-purified proteins of fibronectin and thioredoxin-regulated signal transduction pathways in FIC pathogenesis. These results could provide a promising starting point for novel therapeutic approaches in the future.
Conflict of interest statement
Figures

References
-
- Westropp JL, Buffington CA (2002) In vivo models of interstitial cystitis. J Urol 167: 694–702. - PubMed
-
- Lavelle JP, Meyers SA, Ruiz WG, Buffington CA, Zeidel ML, et al. (2000) Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol Renal Physiol 278: F540–553. - PubMed
-
- Buffington CA, Chew DJ, Woodworth BE (1999) Feline interstitial cystitis. J Am Vet Med Assoc 215: 682–687. - PubMed
-
- Nordling J, Fall M, Hanno P (2011) Global concepts of bladder pain syndrome (interstitial cystitis). World J Urol. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

