Insights into factorless translational initiation by the tRNA-like pseudoknot domain of a viral IRES
- PMID: 23236506
- PMCID: PMC3517527
- DOI: 10.1371/journal.pone.0051477
Insights into factorless translational initiation by the tRNA-like pseudoknot domain of a viral IRES
Abstract
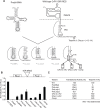
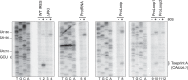
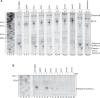
The intergenic region internal ribosome entry site (IGR IRES) of the Dicistroviridae family adopts an overlapping triple pseudoknot structure to directly recruit the 80S ribosome in the absence of initiation factors. The pseudoknot I (PKI) domain of the IRES mimics a tRNA-like codon:anticodon interaction in the ribosomal P site to direct translation initiation from a non-AUG initiation codon in the A site. In this study, we have performed a comprehensive mutational analysis of this region to delineate the molecular parameters that drive IRES translation. We demonstrate that IRES-mediated translation can initiate at an alternate adjacent and overlapping start site, provided that basepairing interactions within PKI remain intact. Consistent with this, IGR IRES translation tolerates increases in the variable loop region that connects the anticodon- and codon-like elements within the PKI domain, as IRES activity remains relatively robust up to a 4-nucleotide insertion in this region. Finally, elements from an authentic tRNA anticodon stem-loop can functionally supplant corresponding regions within PKI. These results verify the importance of the codon:anticodon interaction of the PKI domain and further define the specific elements within the tRNA-like domain that contribute to optimal initiator Met-tRNA(i)-independent IRES translation.
Conflict of interest statement
Figures




References
-
- Pestova TV, Lorsch JR, Hellen CU (2007) The Mechanism of Translation Initiation in Eukaryotes. In: Mathews MB, Sonenberg N, Hershey J, editors. Translational Control in Biology and Medicine. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. 87.
-
- Hellen CU, Sarnow P (2001) Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev 15: 1593–1612. - PubMed
-
- Doudna JA, Sarnow P (2007) Translation Initiation by Viral Internal Ribosome Entry Sites. In: Mathews MB, Sonenberg N, Hershey J, editors. Translation Control in Biology and Medicine. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. 129.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

