Selective development of myogenic mesenchymal cells from human embryonic and induced pluripotent stem cells
- PMID: 23236522
- PMCID: PMC3517512
- DOI: 10.1371/journal.pone.0051638
Selective development of myogenic mesenchymal cells from human embryonic and induced pluripotent stem cells
Abstract
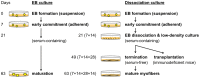
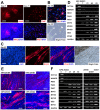
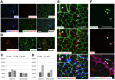
Human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells are promising sources for the cell therapy of muscle diseases and can serve as powerful experimental tools for skeletal muscle research, provided an effective method to induce skeletal muscle cells is established. However, the current methods for myogenic differentiation from human ES cells are still inefficient for clinical use, while myogenic differentiation from human iPS cells remains to be accomplished. Here, we aimed to establish a practical differentiation method to induce skeletal myogenesis from both human ES and iPS cells. To accomplish this goal, we developed a novel stepwise culture method for the selective expansion of mesenchymal cells from cell aggregations called embryoid bodies. These mesenchymal cells, which were obtained by dissociation and re-cultivation of embryoid bodies, uniformly expressed CD56 and the mesenchymal markers CD73, CD105, CD166, and CD29, and finally differentiated into mature myotubes in vitro. Furthermore, these myogenic mesenchymal cells exhibited stable long-term engraftment in injured muscles of immunodeficient mice in vivo and were reactivated upon subsequent muscle damage, increasing in number to reconstruct damaged muscles. Our simple differentiation system facilitates further utilization of ES and iPS cells in both developmental and pathological muscle research and in serving as a practical donor source for cell therapy of muscle diseases.
Conflict of interest statement
Figures

Similar articles
-
Derivation of Skeletal Myogenic Precursors from Human Pluripotent Stem Cells Using Conditional Expression of PAX7.Methods Mol Biol. 2016;1357:423-39. doi: 10.1007/7651_2014_134. Methods Mol Biol. 2016. PMID: 25403466
-
Derivation of stromal (skeletal and mesenchymal) stem-like cells from human embryonic stem cells.Stem Cells Dev. 2012 Nov 20;21(17):3114-24. doi: 10.1089/scd.2012.0035. Epub 2012 Jul 13. Stem Cells Dev. 2012. PMID: 22612317 Free PMC article.
-
Derivation of myogenic progenitors directly from human pluripotent stem cells using a sphere-based culture.Stem Cells Transl Med. 2014 May;3(5):564-74. doi: 10.5966/sctm.2013-0143. Epub 2014 Mar 21. Stem Cells Transl Med. 2014. PMID: 24657962 Free PMC article.
-
Myogenic progenitor specification from pluripotent stem cells.Semin Cell Dev Biol. 2017 Dec;72:87-98. doi: 10.1016/j.semcdb.2017.10.031. Semin Cell Dev Biol. 2017. PMID: 29107681 Free PMC article. Review.
-
Mouse and human pluripotent stem cells and the means of their myogenic differentiation.Results Probl Cell Differ. 2012;55:321-56. doi: 10.1007/978-3-642-30406-4_18. Results Probl Cell Differ. 2012. PMID: 22918815 Review.
Cited by
-
hiPSC-derived iMSCs: NextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine.J Cell Mol Med. 2016 Aug;20(8):1571-88. doi: 10.1111/jcmm.12839. Epub 2016 Apr 21. J Cell Mol Med. 2016. PMID: 27097531 Free PMC article. Review.
-
Effective restoration of dystrophin expression in iPSC Mdx-derived muscle progenitor cells using the CRISPR/Cas9 system and homology-directed repair technology.Comput Struct Biotechnol J. 2020 Mar 25;18:765-773. doi: 10.1016/j.csbj.2020.03.012. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32280431 Free PMC article.
-
Myogenic Differentiation of iPS Cells Shows Different Efficiency in Simultaneous Comparison of Protocols.Cells. 2021 Jul 2;10(7):1671. doi: 10.3390/cells10071671. Cells. 2021. PMID: 34359837 Free PMC article.
-
Directed in vitro myogenesis of human embryonic stem cells and their in vivo engraftment.PLoS One. 2013 Aug 19;8(8):e72023. doi: 10.1371/journal.pone.0072023. eCollection 2013. PLoS One. 2013. PMID: 23977197 Free PMC article.
-
Precise Correction of Disease Mutations in Induced Pluripotent Stem Cells Derived From Patients With Limb Girdle Muscular Dystrophy.Mol Ther. 2016 Apr;24(4):685-96. doi: 10.1038/mt.2016.40. Epub 2016 Feb 26. Mol Ther. 2016. PMID: 26916285 Free PMC article.
References
-
- Wallace GQ, McNally EM (2009) Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol 71: 37–57. - PubMed
-
- Wang YX, Rudnicki MA (2012) Satellite cells, the engines of muscle repair. Nat Rev Mol Cell Biol 13: 127–133. - PubMed
-
- Cerletti M, Shadrach J, Jurga S, Sherwood R, Wagers A (2008) Regulation and Function of Skeletal Muscle Stem Cells. Cold Spring Harb Symp Quant Biol 73: 317–322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

