Ultrasound-mediated vascular gene transfection by cavitation of endothelial-targeted cationic microbubbles
- PMID: 23236976
- PMCID: PMC3682923
- DOI: 10.1016/j.jcmg.2012.05.017
Ultrasound-mediated vascular gene transfection by cavitation of endothelial-targeted cationic microbubbles
Abstract
Objectives: Ultrasound-mediated gene delivery can be amplified by acoustic disruption of microbubble carriers that undergo cavitation. We hypothesized that endothelial targeting of microbubbles bearing cDNA is feasible and, through optimizing proximity to the vessel wall, increases the efficacy of gene transfection.
Background: Contrast ultrasound-mediated gene delivery is a promising approach for site-specific gene therapy, although there are concerns with the reproducibility of this technique and the safety when using high-power ultrasound.
Methods: Cationic lipid-shelled decafluorobutane microbubbles bearing a targeting moiety were prepared and compared with nontargeted microbubbles. Microbubble targeting efficiency to endothelial adhesion molecules (P-selectin or intercellular adhesion molecule [ICAM]-1) was tested using in vitro flow chamber studies, intravital microscopy of tumor necrosis factor-alpha (TNF-α)-stimulated murine cremaster muscle, and targeted contrast ultrasound imaging of P-selectin in a model of murine limb ischemia. Ultrasound-mediated transfection of luciferase reporter plasmid charge coupled to microbubbles in the post-ischemic hindlimb muscle was assessed by in vivo optical imaging.
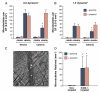
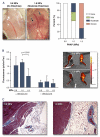
Results: Charge coupling of cDNA to the microbubble surface was not influenced by the presence of targeting ligand, and did not alter the cavitation properties of cationic microbubbles. In flow chamber studies, surface conjugation of cDNA did not affect attachment of targeted microbubbles at microvascular shear stresses (0.6 and 1.5 dyne/cm(2)). Attachment in vivo was also not affected by cDNA according to intravital microscopy observations of venular adhesion of ICAM-1-targeted microbubbles and by ultrasound molecular imaging of P-selectin-targeted microbubbles in the post-ischemic hindlimb in mice. Transfection at the site of high acoustic pressures (1.0 and 1.8 MPa) was similar for control and P-selectin-targeted microbubbles but was associated with vascular rupture and hemorrhage. At 0.6 MPa, there were no adverse bioeffects, and transfection was 5-fold greater with P-selectin-targeted microbubbles.
Conclusions: We conclude that ultrasound-mediated transfection at safe acoustic pressures can be markedly augmented by endothelial juxtaposition.
Copyright © 2012 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
Getting good vibes: the therapeutic power of microbubbles and ultrasound.JACC Cardiovasc Imaging. 2012 Dec;5(12):1263-6. doi: 10.1016/j.jcmg.2012.09.007. JACC Cardiovasc Imaging. 2012. PMID: 23236977 No abstract available.
References
-
- Shohet RV, Chen S, Zhou YT, et al. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation. 2000;101:2554–6. - PubMed
-
- Taniyama Y, Tachibana K, Hiraoka K, et al. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002;105:1233–9. - PubMed
-
- Porter TR, Iversen PL, Li S, Xie F. Interaction of diagnostic ultrasound with synthetic oligonucleotide-labeled perfluorocarbon-exposed sonicated dextrose albumin microbubbles. J Ultrasound Med. 1996;15:577–84. - PubMed
-
- Suzuki J, Ogawa M, Takayama K, et al. Ultrasound-microbubble-mediated intercellular adhesion molecule-1 small interfering ribonucleic acid transfection attenuates neointimal formation after arterial injury in mice. J Am Coll Cardiol. 2010;55:904–13. - PubMed
-
- Leong-Poi H, Kuliszewski MA, Lekas M, et al. Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle. Circ Res. 2007;101:295–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

