The optimal duration of progesterone supplementation in pregnant women after IVF/ICSI: a meta-analysis
- PMID: 23237065
- PMCID: PMC3551800
- DOI: 10.1186/1477-7827-10-107
The optimal duration of progesterone supplementation in pregnant women after IVF/ICSI: a meta-analysis
Abstract
can improve the rates of clinical pregnancy and live birth, but the optimal duration of treatment remains controversial. The objective of this meta-analysis was to investigate the effects of early progesterone cessation on pregnancy outcomes in women undergoing IVF/ICSI.
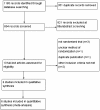
Methods: We searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), the Chinese biomedicine (CBM) literature database, and the Wanfang database. The final search was performed in July 2012. All available randomised trials that compared the effects of early progesterone cessation with progesterone continuation during early pregnancy after IVF/ICSI were included. The main outcome measures were live birth rate, miscarriage rate and ongoing pregnancy rate. Fixed or random-effects models were chosen to calculate the risk ratio (RR).
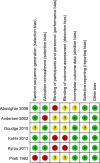
Results: Six eligible studies with a total of 1,201 randomised participants were included in the final analysis. No statistically significant differences were detected between patients who underwent early progesterone cessation and those who received progesterone continuation for luteal phase support in terms of live birth rate (RR: 0.95, 95% CI: 0.86-1.05), miscarriage rate (RR: 1.01, 95% CI: 0.74-1.38) or ongoing pregnancy rate (RR: 0.97, 95% CI: 0.90-1.05). These results did not change after a sensitivity analysis.
Conclusions: The currently available evidence suggests that progesterone supplementation beyond the first positive hCG test after IVF/ICSI might generally be unnecessary, although large-scale randomised controlled trials are needed to strengthen this conclusion.
Figures

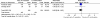


References
-
- Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, Vanderpoel S. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology. Fertil Steril. 2009;92:1520–1524. doi: 10.1016/j.fertnstert.2009.09.009. - DOI - PubMed
-
- Beckers NG, Macklon NS, Eijkemans MJ, Ludwig M, Felberbaum RE, Diedrich K, Bustion S, Loumaye E, Fauser BC. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinising hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J Clin Endocrinol Metab. 2003;88:4186–4192. doi: 10.1210/jc.2002-021953. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

