Population-based versus practice-based recall for childhood immunizations: a randomized controlled comparative effectiveness trial
- PMID: 23237154
- PMCID: PMC3619016
- DOI: 10.2105/AJPH.2012.301035
Population-based versus practice-based recall for childhood immunizations: a randomized controlled comparative effectiveness trial
Abstract
Objectives: We compared the effectiveness and cost-effectiveness of population-based recall (Pop-recall) versus practice-based recall (PCP-recall) at increasing immunizations among preschool children.
Methods: This cluster-randomized trial involved children aged 19 to 35 months needing immunizations in 8 rural and 6 urban Colorado counties. In Pop-recall counties, recall was conducted centrally using the Colorado Immunization Information System (CIIS). In PCP-recall counties, practices were invited to attend webinar training using CIIS and offered financial support for mailings. The percentage of up-to-date (UTD) and vaccine documentation were compared 6 months after recall. A mixed-effects model assessed the association between intervention and whether a child became UTD.
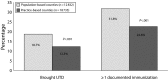
Results: Ten of 195 practices (5%) implemented recall in PCP-recall counties. Among children needing immunizations, 18.7% became UTD in Pop-recall versus 12.8% in PCP-recall counties (P < .001); 31.8% had documented receipt of 1 or more vaccines in Pop-recall versus 22.6% in PCP-recall counties (P < .001). Relative risk estimates from multivariable modeling were 1.23 (95% confidence interval [CI] = 1.10, 1.37) for becoming UTD and 1.26 (95% CI = 1.15, 1.38) for receipt of any vaccine. Costs for Pop-recall versus PCP-recall were $215 versus $1981 per practice and $17 versus $62 per child brought UTD.
Conclusions: Population-based recall conducted centrally was more effective and cost-effective at increasing immunization rates in preschool children.
Trial registration: ClinicalTrials.gov NCT01296906.
Figures
References
-
- Centers for Disease Control and Prevention Ten great public health achievements–United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48(12):241–243 - PubMed
-
- Centers for Disease Control and Prevention Impact of vaccines universally recommended for children–United States, 1990-1998. MMWR Morb Mortal Wkly Rep. 1999;48(12):243–248 - PubMed
-
- McNabb SJ, Jajosky RA, Hall-Baker PAet al.Summary of notifiable diseases—United States, 2005. MMWR Morb Mortal Wkly Rep. 2007;54(53):1–92 - PubMed
-
- US Department of Health and Human Services, Office of Disease Prevention and Health Promotion Healthy People 2020. Available at: http://www.healthypeople.gov/2020/topicsobjectives2020. Accessed February 15, 2012 - PubMed
-
- Briss PA, Rodewald LE, Hinman ARet al.Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18(suppl):97–140 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

