Recognizing and managing the expanded risk of tumor lysis syndrome in hematologic and solid malignancies
- PMID: 23237230
- PMCID: PMC3544586
- DOI: 10.1186/1756-8722-5-75
Recognizing and managing the expanded risk of tumor lysis syndrome in hematologic and solid malignancies
Abstract
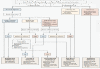
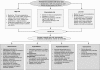
Tumor lysis syndrome (TLS) is widely recognized as a serious adverse event associated with the cytotoxic therapies primarily used in hematologic cancers, such as Burkitt lymphoma and acute lymphoblastic leukemia. In recent years, TLS has been more widely observed, due at least in part to the availability of more effective cancer treatments. Moreover, TLS is seen with greater frequency in solid tumors, and particularly in bulky tumors with extensive metastases and tumors with organ or bone marrow involvement. The consequences of TLS include the serious morbidity and high risk of mortality associated with the condition itself. Additionally, TLS may delay or force an alteration in the patient's chemotherapy regimen. The changing patterns of TLS, as well as its frequency, in the clinical setting, result in unnecessarily high rates of illness and/or fatality. Prophylactic measures are widely available for patients at risk of TLS, and are considered highly effective. The present article discusses the various manifestations of TLS, its risk factors and management options to prevent TLS from occurring.
Figures
References
-
- Cortes J, Moore JO, Maziarz RT, Wetzler M, Craig M, Matous J. et al. Control of plasma uric acid in adults at risk for tumor lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone–results of a multicenter phase III study. J Clin Oncol. 2010;28:4207–4213. doi: 10.1200/JCO.2009.26.8896. - DOI - PMC - PubMed
-
- Wetzstein GA. Tumor lysis syndrome-a treatment guide. Oncology. 2002;5:31–4.
-
- Joshita S, Yoshizawa K, Sano K, Kobayashi S, Sekiguchi T, Morita S. et al. A patient with advanced hepatocellular carcinoma treated with sorafenib tosylate showed massive tumor lysis with avoidance of tumor lysis syndrome. Intern Med. 2010;49:991–994. doi: 10.2169/internalmedicine.49.3153. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

