Low-dose vaporized cannabis significantly improves neuropathic pain
- PMID: 23237736
- PMCID: PMC3566631
- DOI: 10.1016/j.jpain.2012.10.009
Low-dose vaporized cannabis significantly improves neuropathic pain
Abstract
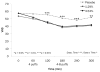
We conducted a double-blind, placebo-controlled, crossover study evaluating the analgesic efficacy of vaporized cannabis in subjects, the majority of whom were experiencing neuropathic pain despite traditional treatment. Thirty-nine patients with central and peripheral neuropathic pain underwent a standardized procedure for inhaling medium-dose (3.53%), low-dose (1.29%), or placebo cannabis with the primary outcome being visual analog scale pain intensity. Psychoactive side effects and neuropsychological performance were also evaluated. Mixed-effects regression models demonstrated an analgesic response to vaporized cannabis. There was no significant difference between the 2 active dose groups' results (P > .7). The number needed to treat (NNT) to achieve 30% pain reduction was 3.2 for placebo versus low-dose, 2.9 for placebo versus medium-dose, and 25 for medium- versus low-dose. As these NNTs are comparable to those of traditional neuropathic pain medications, cannabis has analgesic efficacy with the low dose being as effective a pain reliever as the medium dose. Psychoactive effects were minimal and well tolerated, and neuropsychological effects were of limited duration and readily reversible within 1 to 2 hours. Vaporized cannabis, even at low doses, may present an effective option for patients with treatment-resistant neuropathic pain.
Perspective: The analgesia obtained from a low dose of delta-9-tetrahydrocannabinol (1.29%) in patients, most of whom were experiencing neuropathic pain despite conventional treatments, is a clinically significant outcome. In general, the effect sizes on cognitive testing were consistent with this minimal dose. As a result, one might not anticipate a significant impact on daily functioning.
Published by Elsevier Inc.
Figures
Comment in
-
Marijuana for pain relief: don't jump to conclusions.J Pain. 2013 Oct;14(10):1250-1. doi: 10.1016/j.jpain.2013.07.002. J Pain. 2013. PMID: 24090991 No abstract available.
-
Response to Stacey and Moller's letter to the editor.J Pain. 2013 Oct;14(10):1252-3. doi: 10.1016/j.jpain.2013.07.003. J Pain. 2013. PMID: 24090992 Free PMC article. No abstract available.
References
-
- Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–21. - PubMed
-
- Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: a pilot study. Clinical Pharmacology and Therapeutics. 2007;82:572–8. - PubMed
-
- Backonja MM. Use of anticonvulsants for treatment of neuropathic pain. Neurology. 2002;59:S14–7. - PubMed
-
- Barnes MP. Sativex: clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. Expert Opinion on Pharmacotherapy. 2006;7:607–15. - PubMed
-
- Beaver WT, Buring J, Goldstein A, Johnson K, Jones R. Ad Hoc Group of Experts, Report to the Director. National Institutes of Health; 1997. Workshop on the Medical Utility of Marijuana. p. 19 of 36.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials