A Pictet-Spengler ligation for protein chemical modification
- PMID: 23237853
- PMCID: PMC3538270
- DOI: 10.1073/pnas.1213186110
A Pictet-Spengler ligation for protein chemical modification
Abstract
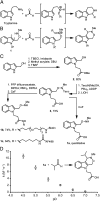
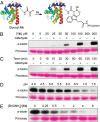
Aldehyde- and ketone-functionalized proteins are appealing substrates for the development of chemically modified biotherapeutics and protein-based materials. Their reactive carbonyl groups are typically conjugated with α-effect nucleophiles, such as substituted hydrazines and alkoxyamines, to generate hydrazones and oximes, respectively. However, the resulting C=N linkages are susceptible to hydrolysis under physiologically relevant conditions, which limits the utility of such conjugates in biological systems. Here we introduce a Pictet-Spengler ligation that is based on the classic Pictet-Spengler reaction of aldehydes and tryptamine nucleophiles. The ligation exploits the bioorthogonal reaction of aldehydes and alkoxyamines to form an intermediate oxyiminium ion; this intermediate undergoes intramolecular C-C bond formation with an indole nucleophile to form an oxacarboline product that is hydrolytically stable. We used the reaction for site-specific chemical modification of glyoxyl- and formylglycine-functionalized proteins, including an aldehyde-tagged variant of the therapeutic monoclonal antibody Herceptin. In conjunction with techniques for site-specific introduction of aldehydes into proteins, the Pictet-Spengler ligation offers a means to generate stable bioconjugates for medical and materials applications.
Conflict of interest statement
Conflict of interest statement: C.R.B. is a cofounder and member of the Scientific Advisory Board of Redwood Bioscience.
Figures



References
-
- Glazer AN. Specific chemical modification of proteins. Annu Rev Biochem. 1970;39:101–130. - PubMed
-
- Wong LS, Khan F, Micklefield J. Selective covalent protein immobilization: Strategies and applications. Chem Rev. 2009;109(9):4025–4053. - PubMed
-
- Shen B-Q, et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol. 2012;30(2):184–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

