Translating advances from the basic biology of aging into clinical application
- PMID: 23237984
- PMCID: PMC3543864
- DOI: 10.1016/j.exger.2012.11.014
Translating advances from the basic biology of aging into clinical application
Abstract
Recently, lifespan and healthspan have been extended in experimental animals using interventions that are potentially translatable into humans. A great deal of thought and work is needed beyond the usual steps in drug development to advance these findings into clinical application. Realistic pre-clinical and clinical trial paradigms need to be devised. Focusing on subjects with symptoms of age-related diseases or frailty or who are at imminent risk of developing these problems, measuring effects on short-term, clinically relevant outcomes, as opposed to long-term outcomes such as healthspan or lifespan, and developing biomarkers and outcome measures acceptable to regulatory agencies will be important. Research funding is a major roadblock, as is lack of investigators with combined expertise in the basic biology of aging, clinical geriatrics, and conducting investigational new drug clinical trials. Options are reviewed for developing a path from the bench to the bedside for interventions that target fundamental aging processes.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
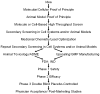
References
-
- Bajada S, Marshall MJ, Wright KT, Richardson JB, Johnson WE. Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone. 2009;45:726–735. - PubMed
-
- Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

