Esophageal epithelial and mesenchymal cross-talk leads to features of epithelial to mesenchymal transition in vitro
- PMID: 23237990
- PMCID: PMC3593809
- DOI: 10.1016/j.yexcr.2012.12.002
Esophageal epithelial and mesenchymal cross-talk leads to features of epithelial to mesenchymal transition in vitro
Abstract
Background: Esophageal fibrosis is a complication of eosinophilic esophagitis (EoE) which has been attributed to both subepithelial fibrosis and to epithelial to mesenchymal transition (EMT), a process by which epithelial cells acquire mesenchymal features. Common to both causes of EoE-fibrosis is the notion that granulocyte-derived TGF-β, induces myofibroblast differentiation of the target cell. To date, the role of esophageal epithelial cells as effector cells in esophageal fibrosis has never been explored. Herein, we investigated consequences of cross-talk between esophageal epithelial cells and fibroblasts, and identified profibrotic cytokines which influence the development of EMT in vitro.
Methods and results: Stimulation of primary fetal esophageal fibroblasts (FEF3) with conditioned media (CEM) from esophageal epithelial cells (EPC2-hTERT), primed FEF3 cells to secrete IL-1β and TNFα, but not TGFβ. To determine whether these cytokines signaled in a paracrine fashion to esophageal epithelial cells, FEF3 cells were stimulated with CEM, followed by transfer of this fibroblast conditioned media (FCM) to EPC2-hTERT cells. Epithelial FCM stimulation increased expression of mesenchymal markers and reduced E-cadherin expression, features of EMT which were TNFα and IL-1β-dependent. Using organotypic culture models, primary EoE epithelial cells exhibited features of EMT compared to non-EoE cells, corresponding to patterns of EMT in native biopsies.
Conclusions: Esophageal epithelial cell and fibroblast cross-talk contributes to esophageal fibrosis. Our results suggest that features of EMT can develop independent of TGF-β and granulocytes, which may have important implications in treatment of EoE.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest: None
Figures
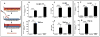
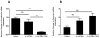




References
-
- Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. The New England journal of medicine. 2004;351(9):940–1. - PubMed
-
- Wada T, Sakai N, Sakai Y, Matsushima K, Kaneko S, Furuichi K. Involvement of bone-marrow-derived cells in kidney fibrosis. Clin Exp Nephrol. 2011;15(1):8–13. - PubMed
-
- Hao H, Gabbiani G, Camenzind E, Bacchetta M, Virmani R, Bochaton-Piallat ML. Phenotypic modulation of intima and media smooth muscle cells in fatal cases of coronary artery lesion. Arterioscler Thromb Vase Biol. 2006;26(2):326–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

