microRNA-21 regulates astrocytic response following spinal cord injury
- PMID: 23238710
- PMCID: PMC3538038
- DOI: 10.1523/JNEUROSCI.3860-12.2012
microRNA-21 regulates astrocytic response following spinal cord injury
Abstract
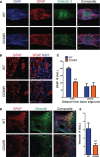
Astrogliosis following spinal cord injury (SCI) involves an early hypertrophic response that serves to repair damaged blood-brain barrier and a subsequent hyperplastic response that results in a dense scar that impedes axon regeneration. The mechanisms regulating these two phases of astrogliosis are beginning to be elucidated. In this study, we found that microRNA-21 (miR-21) increases in a time-dependent manner following SCI in mouse. Astrocytes adjacent to the lesion area express high levels of miR-21 whereas astrocytes in uninjured spinal cord express low levels of miR-21. To study the role of miR-21 in astrocytes after SCI, transgenic mice were generated that conditionally overexpress either the primary miR-21 transcript in astrocytes or a miRNA sponge designed to inhibit miR-21 function. Overexpression of miR-21 in astrocytes attenuated the hypertrophic response to SCI. Conversely, expression of the miR-21 sponge augmented the hypertrophic phenotype, even in chronic stages of SCI recovery when astrocytes have normally become smaller in size with fine processes. Inhibition of miR-21 function in astrocytes also resulted in increased axon density within the lesion site. These findings demonstrate a novel role for miR-21 in regulating astrocytic hypertrophy and glial scar progression after SCI, and suggest miR-21 as a potential therapeutic target for manipulating gliosis and enhancing functional outcome.
Conflict of interest statement
The authors declare no competing financial interests.
Figures







References
-
- Barnabé-Heider F, Frisén J. Stem cells for spinal cord repair. Cell stem cell. 2008;3:16–24. - PubMed
-
- Barnabé-Heider F, Göritz C, Sabelström H, Takebayashi H, Pfrieger FW, Meletis K, Frisén J. Origin of new glial cells in intact and injured adult spinal cord. Cell stem cell. 2010;7:470–482. - PubMed
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
